Retrospective Evaluation of Drug-Drug Interactions With Erlotinib and Gefitinib Use in the Military Health System
Background: Erlotinib and gefitinib are epidermal growth factor receptor–tyrosine kinase inhibitors approved for non–small cell lung cancer treatment by the US Food and Drug Administration. Drug-drug interactions (DDIs) with these agents are vague and poorly understood. Because DDIs can have an effect on clinical outcomes, we aimed to identify drugs that interact with erlotinib or gefitinib and describe their clinical manifestations.
Methods: A retrospective analysis was performed on the health records of patients in the US Department of Defense Cancer Registry (retrieved September 2021), Comprehensive Ambulatory/Professional Encounter Records, and Pharmacy Data Transaction Service database (both retrieved May 2022). Patients’ medical history, diagnoses, and demographics were extracted and analyzed for differences in adverse effects when these agents were used alone vs concomitantly with other prescription drugs. Patients’ diagnoses and prescription drug use were extracted to compare completed vs discontinued treatment groups, identify medications commonly co-administered with erlotinib or gefitinib, and evaluate DDIs with antidepressants.
Results: Of 387 patients using erlotinib, 264 completed treatments; 28 of 33 patients using gefitinib completed treatment. The P value for erlotinib discontinuation when used alone vs concomitantly was < .001, and the P value for gefitinib discontinuation was .06. Patients who took erlotinib or gefitinib concomitantly with a greater number of prescription drugs had a higher rate of treatment discontinuation than those who received fewer medications. Patients in the completed group received 1 to 75 prescription drugs, and those in the completed group were prescribed 3 to 103. Those who discontinued treatment had more diagnosed medical issues than those who completed treatment.
Conclusions: This review cannot conclude that concomitant use with prescription drug(s) resulted in erlotinib or gefitinib discontinuation. There were no significant DDIs determined between erlotinib or gefitinib and antidepressants.
Physicians’ notes on AEs were not detailed in most cases. Notes for gastrointestinal effects, life-altering pruritis, intolerance, peripheral vascular disease, pneumonitis, and progressive disease described the change in status or appearance of a new medical condition but did not indicate whether erlotinib or gefitinib caused the changes or worsened a pre-existing condition.
The causes of AEs were not described in the available notes or the databases. This retrospective data analysis only focused on identifying drugs involved with erlotinib and gefitinib treatment; further mapping of DDIs among patients experiencing AEs needs to be performed, then in vitro data testing before researchers can reach a conclusion.
DDIs With Antidepressants
We used the PDTS database to evaluate patients who experienced AEs, excluding patients who switched treatment. Thirteen patients filled a prescription for erlotinib and reported taking 220 cancer and noncancer prescription drugs. One patient (pruritis) was taking gefitinib along with 16 noncancer prescription drugs.
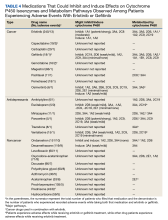
Selective serotonin reuptake inhibitors and other antidepressants have been implicated in CYP 2D6 inhibition and DDIs.48,49 Losartan is a widely used antihypertensive drug with a favorable DDI profile
Our data showed that 16 antidepressants (amitriptyline, bupropion, citalopram, desvenlafaxine, duloxetine, escitalopram, imipramine, fluoxetine, fluvoxamine, mirtazapine, nortriptyline, paroxetine, phenelzine, sertraline, trazodone, and venlafaxine) were recorded with concomitant erlotinib or gefitinib from initiation to completion of therapy or a buffer of 6 months from the first diagnosis date. Based on the date dispensed and days’ supply, only escitalopram could be used in combination with gefitinib treatment. The one patient who filled a prescription for gefitinib and escitalopram completed treatment without recorded AEs. PDTS database confirmed that patients experienced AEs with 5 antidepressants (amitriptyline, mirtazapine, paroxetine, trazodone, and venlafaxine) with concomitant erlotinib use.
Based on the date dispensed and days’ supply, only trazodone could be used in combination with erlotinib. PDTS database showed that cancer drugs (erlotinib and megestrol) and 39 noncancer drugs (including acetaminophen, azithromycin, dexamethasone, hydrocortisone, and polyethylene glycol) were filled by 1 patient whose physician noted skin rash. Another limitation of using databases to reflect clinical practice is that although megestrol is listed as a cancer drug by code in the PDTS database, it is not used for nonendometrial or gynecologic cancers. However, because of the PDTS database classification, megestrol is classified as a cancer drug in this retrospective review.
This retrospective review found no significant DDIs for erlotinib or gefitinib, with 1 antidepressant taken by 1 patient for each respective treatment. The degree of inhibition and induction for escitalopram and trazodone are categorized as weak, minimal, or none; therefore, while 1:1 DDIs might be little or no effect, 1:1:1 combination DDIs could have a different outcome. This retrospective data collection cannot be linked to the in vitro hepatocyte DDIs from erlotinib and gefitinib in previous studies.51,52