Physician Responsiveness to Positive Blood Culture Results at the Minneapolis Veterans Affairs Hospital—Is Anyone Paying Attention?
Background: Patients presenting with suspected infections are typically placed on empiric broad-spectrum antibiotics. With mounting evidence supporting the efficacy of using the narrowest spectrum of antimicrobial therapy to cover the suspected pathogen, current guidelines recommend decreasing the breadth of coverage in response to culture results both in relation to microbe identification and antibiotic sensitivity.
Methods: A retrospective chart review of electronic health records at the Minneapolis Veterans Affairs Medical Center (VAMC) in Minnesota was performed for 208 positive blood cultures with antibiotic spectrum analysis from July 1, 2015 to June 30, 2016. The time of reporting for pathogen identification and subsequent pathogen susceptibilities were compared to the time at which any alterations to antibiotic coverage were made. The breadth of antibiotic coverage was recorded using a nonlinear spectrum score. The use of this score allowed for the reliable classification of antibiotic adjustments as either deescalation, escalation, or no change.
Results: The percentage of cases deescalated was higher in response to physician (house staff or attending physician) notification of pathogen susceptibility information when compared with a response to pathogen identification alone (33.2% vs 22.6%). Empiric antibiotics were not altered within 24 hours in response to pathogen identification in 70.7% of cases and were not altered within 24 hours in response to pathogen sensitivity determination in 58.6% of cases. However, when considering the time frame from when empiric antibiotics were started to 24 hours after notification of susceptibility information, 49.5% of cases were deescalated and 41.5% of cases had no net change in the antibiotic spectrum score. The magnitude of deescalations were notably larger than escalations. The mean (SD) time to deescalation of antibiotic coverage was shorter ( P =.049) in response to pathogen identification at 8 (7.4) hours compared with sensitivity information at 10.4 (7) hours, but may not be clinically relevant.
Conclusion: Health care providers at the Minneapolis VAMC appear to be using positive blood culture results in a timely fashion consistent with best practices. Because empirically initiated antibiotics typically are broad in spectrum, the magnitude of deescalations were notably larger than escalations. Adherence to these standards may be a reflection of the infectious disease staff oversight of antibiotic administration. Furthermore, the systems outlined in this quality improvement study may be replicated at other VAMCs across the country by either in-house infectious disease staff or through remote monitoring of the electronic health record by other infectious disease experts at a more centralized VAMC. Widespread adoption throughout the Veterans Health Administration may result in improved antibiotic resistance profiles and better clinical outcomes for our nation’s veterans
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
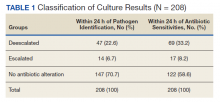
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.