Outcomes Comparison of the Veterans’ Choice Program With the Veterans Affairs Health Care System for Hepatitis C Treatment
Background: The US Department of Veterans Affairs (VA) has been stressed by the large number of veterans requiring direct-acting antiviral (DAA) medications for hepatitis C virus (HCV) treatment. The Veterans Choice Program provides VA patients more options to receive treatment. This study compared the experience of veterans who received HCV treatment through the Veterans Choice Program and those that received treatment at the VA Loma Linda Healthcare System (VALLHCS) in fiscal year (FY) 2016.
Methods: A chart review was performed on all veterans referred by VALLHCS to Choice for HCV treatment during FY 2016, and matched to veterans who received treatment at VALLHCS. Data collected included Fibrosis-4 score (Fib-4), platelet count, days elapsed between time of referral and time of appointment (wait time), rate of sustained virologic response at 12 weeks (SVR12), reason for treatment failure, and cost effectiveness.
Results: One hundred veterans were referred to Choice; 71 were seen at least once by a Choice provider, and 61 completed a treatment course. Mean Fib-4 and platelet count was 1.9 and 228,000 for the Choice population and 3.4 and 158,000 for the VALLHCS population, respectively. There was no difference in SVR12 rate. Mean wait time was 42 days for Choice vs 29 days for VALLHCS (P < .001). Choice health care providers incurred a mean $8,561.40 in additional costs per veteran seen.
Conclusions: While treatment success rates were similar between Choice and VALLHCS, the degree of liver fibrosis was more advanced in the VALLHCS population. The wait time for care was longer with Choice compared with a direct referral within the VA. While Choice offers a potential solution to providing care for veterans, the current program has unique problems that must be considered.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
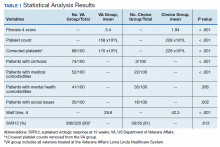
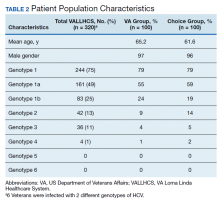
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).