Analysis of Pharmacist Interventions Used to Resolve Safety Target of Polypharmacy (STOP) Drug Interactions
Background: Statin drug interactions commonly increase the risk of muscle-related toxicities. The medical literature supports consultative pharmacist interventions to resolve drug interactions, but studies demonstrating autonomous pharmacist interventions are lacking.
Objective: To evaluate the complementary impact of using pharmacist-led protocols and pharmacists with prescriptive authority to resolve statin drug interactions.
Methods: Pharmacist-led protocols were developed to address gemfibrozil-statin and niacin-statin interactions. Pharmacists discontinued gemfibrozil and niacin by protocol or referred patients to the Patient Aligned Care Team (PACT) Pharmacy Clinic for individualized management. After all drug interactions were addressed, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of gemfibrozil and niacin discontinuation by protocol on patients’ triglyceride (TG) laboratory results. The coprimary endpoints were the change in TGs and the percentage of patients with TGs ≥ 500 mg/dL, following pharmacist discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT Clinical Pharmacy Specialists' (CPS) pharmacologic interventions.
Results: The gemfibrozil and niacin protocols addressed 397 drug interactions. Seventy-six patients underwent gemfibrozil discontinuation by protocol and had TG laboratory results available. TG levels decreased or increased by < 100 mg/dL for 62 patients (82%), and 1 patient (1.3%) experienced TG elevation above the threshold of 500 mg/dL. Thirty-six patients had niacin discontinued by protocol and available laboratory results. The TG levels decreased or increased by < 100 mg/dL for 33 patients (91.7%), and no patients had TG levels increase above the threshold of 500 mg/dL. The mean time required to resolve both gemfibrozil and niacin drug interactions was 15.5 minutes per patient. A total of 129 patients were referred to the PACT Pharmacy Clinic to manage gemfibrozil and niacin drug interactions. TG laboratory results were available for 80 gemfibrozil patients (74.8%) and 16 niacin patients (72.7%). The PACT CPS made 171 pharmacologic interventions to address drug interactions and the median of 2 encounters per patient.
Conclusions: This single-site quality improvement analysis supports the complementary use of protocols and pharmacists with prescriptive authority to resolve statin drug interactions. These data support expanded roles for pharmacists, across settings, to mitigate select drug interactions.
Following all pharmacist interventions, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of discontinuing gemfibrozil and niacin by protocol on patients’ laboratory results. The coprimary endpoints were to describe the change in TG levels and the percentage of patients with TGs ≥ 500 mg/dL at least 5 weeks following the pharmacist-directed discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT CPS pharmacologic interventions. Additionally, a quality assurance peer review was used to ensure the pharmacists appropriately utilized the protocols.
Data were collected from August 2016 to September 2017 for patients prescribed gemfibrozil and from May 2017 to January 2018 for patients prescribed niacin. The time spent resolving interactions was quantified based on encounter data. Descriptive statistics were used to analyze demographic information and the endpoints associated with each outcome. The project was reviewed by the University of Missouri Institutional Review Board, Truman VA privacy and information security officers, and was determined to meet guidelines for quality improvement.
Results
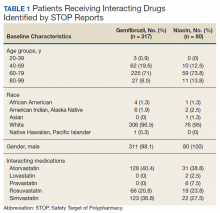
The original STOP report included 397 drug interactions involving statins with gemfibrozil or niacin (Table 1). The majority of patients were white and male aged 60 to 79 years. Gemfibrozil was the most common drug involved in all interactions (79.8%). The most common statins were atorvastatin (40%) and simvastatin (36.5%).
Gemfibrozil-Statin Interactions
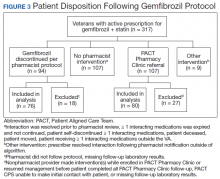
Pharmacists discontinued gemfibrozil by protocol for 94 patients (29.6%), and 107 patients (33.8%) were referred to the PACT Pharmacy Clinic (Figure 3). For the remaining 116 patients (36.6%), the drug interaction was addressed outside of the protocol for the following reasons: the drug interaction was resolved prior to pharmacist review; an interacting prescription was expired and not to be continued; the patient self-discontinued ≥ 1 interacting medications; the patient was deceased; the patient moved; the patient was receiving ≥ 1 interacting medications outside of the VA; or the prescriber resolved the interaction following notification by the pharmacist.
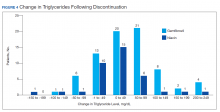
Ultimately, the interaction was resolved for all patients with a gemfibrozil-statin interaction on the STOP report. Following gemfibrozil discontinuation by protocol, 76 patients (80.9%) had TG laboratory results available and were included in the analysis. Sixty-two patients’ (82%) TG levels decreased or increased by < 100 mg/dL (Figure 4), and the TG levels of 1 patient (1.3%) increased above the threshold of 500 mg/dL. The mean (SD) time to the first laboratory result after the pharmacists mailed the notification letter was 6.5 (3.6) months (range, 1-17). The pharmacists spent a mean of 16 minutes per patient resolving each interaction.