Analysis of Pharmacist Interventions Used to Resolve Safety Target of Polypharmacy (STOP) Drug Interactions
Background: Statin drug interactions commonly increase the risk of muscle-related toxicities. The medical literature supports consultative pharmacist interventions to resolve drug interactions, but studies demonstrating autonomous pharmacist interventions are lacking.
Objective: To evaluate the complementary impact of using pharmacist-led protocols and pharmacists with prescriptive authority to resolve statin drug interactions.
Methods: Pharmacist-led protocols were developed to address gemfibrozil-statin and niacin-statin interactions. Pharmacists discontinued gemfibrozil and niacin by protocol or referred patients to the Patient Aligned Care Team (PACT) Pharmacy Clinic for individualized management. After all drug interactions were addressed, a retrospective quality improvement analysis was conducted. The primary outcome was to evaluate the impact of gemfibrozil and niacin discontinuation by protocol on patients’ triglyceride (TG) laboratory results. The coprimary endpoints were the change in TGs and the percentage of patients with TGs ≥ 500 mg/dL, following pharmacist discontinuation by protocol. Secondary outcomes included the time required to resolve the interactions and a description of the PACT Clinical Pharmacy Specialists' (CPS) pharmacologic interventions.
Results: The gemfibrozil and niacin protocols addressed 397 drug interactions. Seventy-six patients underwent gemfibrozil discontinuation by protocol and had TG laboratory results available. TG levels decreased or increased by < 100 mg/dL for 62 patients (82%), and 1 patient (1.3%) experienced TG elevation above the threshold of 500 mg/dL. Thirty-six patients had niacin discontinued by protocol and available laboratory results. The TG levels decreased or increased by < 100 mg/dL for 33 patients (91.7%), and no patients had TG levels increase above the threshold of 500 mg/dL. The mean time required to resolve both gemfibrozil and niacin drug interactions was 15.5 minutes per patient. A total of 129 patients were referred to the PACT Pharmacy Clinic to manage gemfibrozil and niacin drug interactions. TG laboratory results were available for 80 gemfibrozil patients (74.8%) and 16 niacin patients (72.7%). The PACT CPS made 171 pharmacologic interventions to address drug interactions and the median of 2 encounters per patient.
Conclusions: This single-site quality improvement analysis supports the complementary use of protocols and pharmacists with prescriptive authority to resolve statin drug interactions. These data support expanded roles for pharmacists, across settings, to mitigate select drug interactions.
Statins are one of the most common medications dispensed in the US and are associated with clinically significant drug interactions.1,2 The most common adverse drug reaction (ADR) of statin drug interactions is muscle-related toxicities.2 Despite technology advances to alert clinicians to drug interactions, updated statin manufacturer labeling, and guideline recommendations, inappropriate prescribing and dispensing of statin drug interactions continues to occur in health care systems.2-10
The medical literature has demonstrated many opportunities for pharmacists to prevent and mitigate drug interactions. At the points of prescribing and dispensing, pharmacists can reduce the number of potential drug interactions for the patient.11-13 Pharmacists also have identified and resolved drug interactions through quality assurance review after dispensing to a patient.7,8
Regardless of the time point of an intervention, the most common method pharmacists used to resolve drug interactions was through recommendations to a prescriber. The recommendations were generated through academic detailing, clinical decision support algorithms, drug conversions, or the pharmacist’s expertise. Regardless of the method the pharmacist used, the prescriber had the final authority to accept or decline the recommendation.7,8,11-13 Although these interventions were effective, pharmacists could further streamline the process by autonomously resolving drug interactions. However, these types of interventions are not well described in the medical literature.
Background
The US Department of Veterans Affairs (VA) Veterans Integrated Service Network (VISN), established the Safety Target of Polypharmacy (STOP) report in 2015. At each facility in the network, the report identified patients who were dispensed medications known to have drug interactions. The interactions were chosen by the VISN, and the severity of the interactions was based on coding parameters within the VA computerized order entry system, which uses a severity score based on First Databank data. At the Harry S. Truman Memorial Veterans’ Hospital (Truman VA) in Columbia, Missouri, > 500 drug interactions were initially active on the STOP report. The most common drug interactions were statins with gemfibrozil and statins with niacin.14-18 The Truman VA Pharmacy Service was charged with resolving the interactions for the facility.
The Truman VA employs 3 Patient Aligned Care Team (PACT) Clinical Pharmacy Specialists (CPS) practicing within primary care clinics. PACT is the patientcentered medical home model used by the VA. PACT CPS are ambulatory care pharmacists who assist providers in managing diseases using a scope of practice. Having a scope of practice would have allowed the PACT CPS to manage drug interactions with independent prescribing authority. However, due to the high volume of STOP report interactions and limited PACT CPS resources, the Pharmacy Service needed to develop an efficient, patient-centered method to resolve them. The intervention also needed to allow pharmacists, both with and without a scope of practice, to address the interactions.
Methods
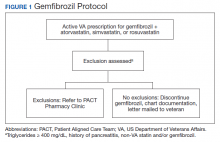
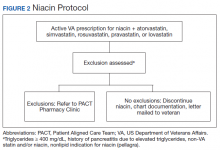
The Truman VA Pharmacy Service developed protocols, approved by the Pharmacy and Therapeutics (P&T) Committee, to manage the specific gemfibrozil-statin and niacinstatin interactions chosen for the VISN 15 STOP report (Figures 1 and 2). The protocols were designed to identify patients who did not have a clear indication for gemfibrozil or niacin, were likely to maintain triglycerides (TGs) < 500 mg/dL without these medications, and would not likely require close monitoring after discontinuation.19 The protocols allowed pharmacists to autonomously discontinue gemfibrozil or niacin if patients did not have a history of pancreatitis, TGs ≥ 400 mg/dL or a nonlipid indication for niacin (eg, pellagra) after establishing care at Truman VA. Additionally, both interacting medications had to be dispensed by the VA. When pharmacists discontinued a medication, it was documented in a note in the patient electronic health record. The prescriber was notified through the note and the patient received a notification letter. Follow-up laboratory monitoring was not required as part of the protocol.
If patients met any of the exclusion criteria for discontinuation, the primary care provider (PCP) was notified to place a consult to the PACT Pharmacy Clinic for individualized interventions and close monitoring. Patients prescribed niacin for nonlipid indications were allowed to continue with their current drug regimen. At each encounter, the PACT CPS assessed for ADRs, made individualized medication changes, and arranged follow-up appointments. Once the interaction was resolved and treatment goals met, the PCP resumed monitoring of the patient’s lipid therapy.