Assessment of IV Edaravone Use in the Management of Amyotrophic Lateral Sclerosis
Background: Edaravone has been shown to slow functional degeneration of amyotrophic lateral sclerosis (ALS). The primary objective of this study was to assess ALS disease progression in veterans on IV edaravone compared with veterans who received standard of care.
Methods: This retrospective case-control study was conducted at a large, academic US Department of Veteran Affairs medical center. The primary endpoint was the change in baseline Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) scores after 6 months of IV edaravone compared with standard-of-care ALS management. The secondary outcomes included change in ALSFRS-R scores, percent forced vital capacity (%FVC) and speech intelligibility stage (SIS) 3 to 24 months after initiation of therapy, duration of edaravone completed (months), time to death (months), and safety outcomes.
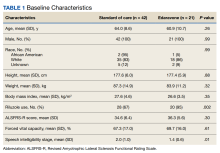
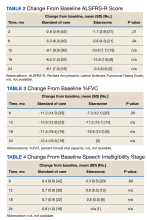
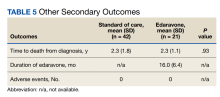
Results: Twenty-one edaravone and 42 standard-of-care patients were evaluated. No difference was noted in ALSFRS-R at 6 months between the edaravone and standard-of-care groups (P = .84). Additionally, no difference was noted in change from baseline %FVC, change from baseline SIS, and time to death between the 2 groups (P > .05). No safety events were reported in either group.
Conclusions: No difference was noted in the rate of ALS disease progression between patients who received IV edaravone vs standard of care.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
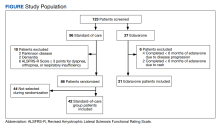
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.