Preoperative Insulin Intensification to Improve Day of Surgery Blood Glucose Control
Background: Guidelines offer varying recommendations for preoperative long-acting basal insulin dosing, despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL.
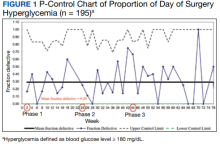
Observations: We iteratively adjusted health care practitioner (HCP) instructions to intensify insulin dosing on the evening before surgery for 195 consecutive patients with diabetes mellitus treated with long-acting basal insulin with an evening dosage. Baseline data was collected in phase 1. In phase 2, the preoperative insulin dose on the evening before surgery was increased for patients with hemoglobin A 1c (HbA 1c ) > 8%; in phase 3, it was increased for patients with HbA 1c ≤ 8% while sustaining the phase 2 change. Increased preoperative insulin doses did not change the rates of day of surgery (DOS) hyperglycemia or hypoglycemia. Overall, HCP adherence to the modified protocols was high (89%). A decline in HCP adherence after phase 2 protocol change was associated with a transient increase in DOS hyperglycemia. Patient adherence to preoperative medication instructions was high (86%) and was not affected by protocol changes.
Conclusions: Preoperative insulin intensification the evening before surgery did not change rates of DOS hyperglycemia or hypoglycemia. HCP adherence decreased transiently, which briefly increased DOS hyperglycemia rates in some patients.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
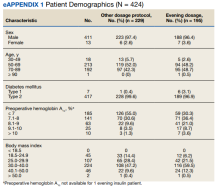
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening