A Practical Guide to Urine Drug Monitoring
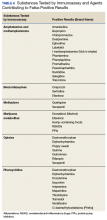
False positives. Due to the lack of specificity of UDM by IA, false positives are common; with the exception of cocaine. Clinicians must obtain a comprehensive medication history of the patient, including over-the-counter medications, herbals, and supplements. Table 6 lists common sources of false positives with UDM by IA.1,8,9
False negatives. A variety of factors can cause false-negative results, includingthe cross-reactivity of the antibody in the IA, the cutoff concentration that yields a positive result, and/or the time between drug ingestion. As discussed previously, the opiate panel tests for metabolites of morphine, codeine, and heroin, which consequently may lead to semisynthetic/synthetic opioids not being detected.8,11 For example, a patient who was prescribed hydrocodone/acetaminophen 5 mg/325 mg 4 times a day, tests negative for opiates by IA. The negative result is not unexpected because the dose of semisynthetic opioid is too low for detection by IA.
Chromatography
Chromatography generally is reserved for confirmatory or definitive testing when the initial UDM by IA results are unexpected.1 Unlike IA, chromatography can detect the presence of specific drugs and/or metabolites. Types of chromatography testing include GC/MS, liquid chromatography tandem mass spectrometry (LC/MS/MS), and high-performance liquid chromatography.9 Depending on the specific test, chromatography uses a gas or liquid carrier medium to separate the urine sample’s compounds by their molecular interactions with the carrier medium (mainly by different polarities). During this separation process, all the individual compounds are fed into a mass spectrometer, that ionizes the compounds and detects fragments by using their mass-to-charge ratios. This process allows for the identification of distinct compounds based on their molecular fingerprints.
Gas chromatography/mass spectrometry has remained the standard test for confirmatory testing.1,8 However, it is important to note that LC/MS/MS has been gaining favor over GC/MS. Using LC/MS/MS requires less urine volume to conduct an analysis, and the analysis has a second analytical separation step, thus it is expected to have a lower susceptibility to false results caused by concomitant use of other medications.15,16
Regardless of the test medium, quantitative confirmation through chromatography offers several advantages over IA. It is more accurate, as it can identify small quantities of specific drugs and confirm their presence in urine.8 Also, although there are still cutoff limits associated with chromatography, the specific cutoffs are much lower in value than those in IA tests.Finally, a study conducted in 2010 by Pesce and colleagues found that IA testing was associated with varying rates of false-negative results compared with those of LC-MS/MS.17 Specifically, false-negative rates associated with IA were found to be 22%, 50%, and 23.4% for benzodiazepines, cocaine, and propoxyphene, respectively.17 Unfortunately, chromatography testing methods take longer to produce results and are costly compared with those of IA.Thus, chromatography testing methods typically are reserved for when the IA produces unexpected results. Conversely, IA can be done at point of care with in-office readable cups or strips, or sent out for a 24-hour to 48-hour turnaround time.7,8
Alcohol Testing
Health care providers also could screen for alcohol misuse, which can compromise safe opioid use. Alcohol can accelerate the release of certain sustained-release formulations, causing “dose dumping.”18 Furthermore, alcohol also can increase the risk of opioid-induced respiratory depression. Many laboratories include ethanol that is measured using an enzymatic reaction and generally detected 12 hours after alcohol use.7-9 Urinary ethanol is not an optimal marker for assessing alcohol use. Ethyl glucuronide (EtG) and ethyl sulfate (EtS) are 2 minor metabolites of ethanol formed by UDP-glucuronosyltransferase.19 These markers can be detected for up to 80 hours after alcohol consumption. Markers for prolonged and/or heavy drinking include but are not limited to phosphatidylethanol, γ-glutamyltransferase, or carbohydrate-deficient transferrin.20
Pharmacokinetics/Pharmacogenetics
Pharmacokinetics is what the body does with the drug and is measured by absorption, distribution, metabolism, and elimination.16 Pharmacokinetics ultimately determines the fate of how much and how fast a drug and/or metabolites end up in the urine. It is important to understand the pharmacokinetics to interpret the results of UDM by chromatography as the reported results include parent drugs and metabolites.
Some metabolites of medications available commercially could be mistaken as if the patient were taking a medication that was not prescribed. For example, hydromorphone is a metabolite of hydrocodone and oxymorphone is a metabolite of oxycodone, both of which are commercially available as stand-alone prescriptions. Likewise, oxazepam is commercially available as is temazepam, and both are metabolites of diazepam. Also, it is important to consider patient’s body habitus, which affects volume of distribution, meaning more drug is stored in the periphery and may have a longer detection window.21 Patients with renal and/or hepatic impairment can have reduced clearance of the medications.
It is equally important to consider the role that pharmacogenetic polymorphism can play in UDM, as polymorphisms may impact results.1,8 For example, consider a patient on extended-release oxycodone 30 mg twice daily. Oxycodone is metabolized via cytochrome (CYP) P450 enzyme 3A4 into noroxycodone and, to a much lesser extent, by CYP2D6 into oxymorphone. In this case, if tested by chromatography, the patient’s urine level of oxycodone should be higher than that of either metabolite; specifically, the urine level of noroxycodone should be higher than that of oxymorphone. If there are only concentrations of oxycodone found in the urine with no metabolites, the possible explanations are either the patient dissolved oxycodone into the urine sample without ingestion or the patient may have poor activity of CYP2D6 and CYP3A4 isoenzymes; the latter of which can be confirmed by pharmacogenetic testing. Notwithstanding, drug-drug interactions with CYP inhibitors can produce the same outcome.