A Practical Guide to Urine Drug Monitoring
Urine drug monitoring (UDM) is an important tool to screen adherence and identify possible misuse and abuse in patients on opioid therapy.1 Various guidelines for opioid therapy emphasize the importance of UDM as a standard of care.2-6 Routine and random monitoring is recommended for all patients on long-term opioid therapy prior to initiation and throughout duration of therapy.1-3 The recommended UDM frequency varies based on individual risk assessment and clinical judgment. Similar to any other diagnostic or monitoring test, the goal for UDM should be to guide therapy and improve patient care (Box). Inappropriate interpretation of the results and failure to order definitive testing when necessary may adversely affect patient care.
Urine Drug Monitoring
Sample Collection
Urine drug testing generally requires a minimum of 30 mL of urine (depending on the kit type) collected in a private restroom. In the authors’ experience, the sample collection most often is unobserved in clinical practice. Most laboratories keep urine samples for a limited time, often 7 days. Therefor, if results are unexpected, health care providers must notify the laboratory in a timely manner to order definitive testing if indicated.
Specimen Validity Testing
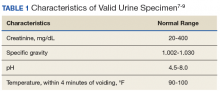
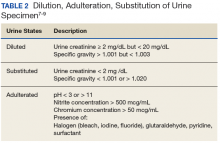
Attempts to dilute, adulterate, and substitute urine may be detected by visual inspection and laboratory validity testing. Validity testing of urine specimens includes temperature, specific gravity, pH, urine creatinine, and presence of adulterants (Tables 1 and 2).7-9
The combination of specific gravity and urinary creatinine may help screen for dilution or substitution. Dilution may occur precollection by consumption of excess amounts of fluids or postcollection by adding fluid to the specimen. Other causes of diluted urine should be considered, such as renal tubular dysfunction or diuretic use. Household adulterants include vinegar, detergent, sodium chloride, hydrogen peroxide, eye/nose drops, soda, or ammonia.10 There are numerous commercially available adulterants, including Klear, UrinAid, Urine Luck, Stealth Synthetics, Whizzies, and Clear Choice. The active ingredients of some include peroxide/peroxidase, sodium or potassium nitrate, pyridinium chlorochromate, or glutaraldehyde. There are laboratory tests to detect the presence of these adulterants. Whenever in doubt, it is advisable that health care providers (HCPs) contact their laboratory to investigate tampering. Another approach if tampering is suspected is to collect blood samples. Although this method is more expensive and invasive, it eliminates means of tampering. Hair follicle testing is an option as well.
Types of Urine Drug Monitoring
There are 2 general types of UDM: Presumptive by immunoassay (IA) and confirmatory testing by chromatography. Simply, UDM by IA commonly referred to as urine drug screening (UDS), serves as the differential assessments, whereas chromatography is the definitive assessment. This article reviews the clinical utility and limitations of the 2 types of UDM, including false positives and false negatives, and when to order more tests.
Immunoassay
The IA drug test uses antibodies to detect the presence of selected drugs and/or their metabolites based on a predetermined cutoff threshold.8 Immunoassay monitoring is the initial qualitative test to identify the presence of drug classes in the urine based on a detection threshold. Typically, UDM by IA is performed as an initial evaluation of potential appropriate use, misuse, nonuse, or abuse of medications. It also can detect the presence of illegal substances or unprescribed medications. Immunoassay is relatively quick, inexpensive, and sensitive; however, because it lacks specificity, it can result in various false positives and false negatives.
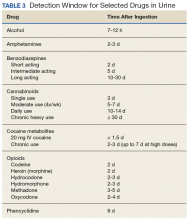
Immunoassay tests also are subject to varying windows of detection depending on the substance ingested (Table 3).
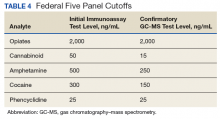
The cutoff levels listed in Table 1 are consistent with testing for employment but not necessarily for aberrant behavior in patients receiving long-term opioid therapy. These cutoffs lower the risk of false positives and provide better accuracy with clinical monitoring. For example, a level of 2,000 ng/mL is listed for both test types in Table 4, but for clinical testing, the IA cutoff is 3,000 ng/mL, and gas chromatography/mass spectrometry (GC-MS) can detect even trace amounts of opioid and their metabolites.
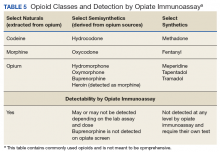
The opiate panel with IA tests for opium alkaloids and/or their metabolites, including morphine and codeine.7-9 Heroin is a semisynthetic opioid that is metabolized to diacetyl morphine and ultimately is detected as morphine.7,8 Other semisynthetic opioids, such as hydrocodone and oxycodone, may or may not be detected by the opiate IA depending on the dose and assay.
Benzodiazepine IAs often are designed to detect nordiazepam, oxazepam, and temazepam, all of which are metabolites of diazepam. However, benzodiazepine IAs also can detect other drugs that are structurally similar to benzodiazepines.11,12 This means that benzodiazepines are detected based on their ability to cross-react with the IA test. Lorazepam and clonazepam have low cross-reactivity and are generally not detected on benzodiazepine IA.12,13 Therefore, it is not uncommon for patients on lorazepam or clonazepam to test negative for benzodiazepines on this IA. If these patients do test positive at low doses, it could be a concern that they are taking a different benzodiazepine instead of, or in addition to, the prescribed medication.
Amphetamines and methamphetamine are simple molecules that are difficult to develop specific antibodies for; therefore, they carry a high false-positive rate with IA testing.8 It is important to note that methylphenidate is not detected by the amphetamine IA as it is not an amphetamine.8 The IA for cocaine tests specifically for benzoylecgonine, a metabolite specific to cocaine and has no cross-reactivity.8,12,14