HYSTEROSCOPIC STERILIZATION
New devices do not require abdominal access or general anesthesia, and offer rapid recovery
IN THIS ARTICLE
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
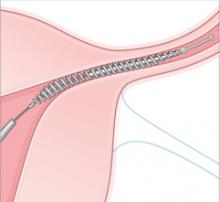
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
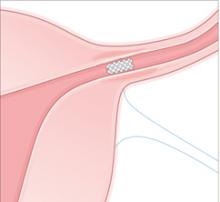
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
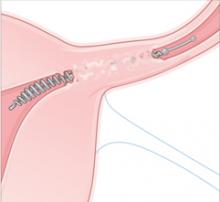
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.