2016 Update on cervical disease
The future of treatment for cervical cancer involves therapeutic vaccines and T-cell therapy. What you should know. Plus, follow-up data on HPV primary screening.
In this article
• The success of adoptive T-cell therapy
• Long-term follow-up of primary HPV screening
Adoptive T-cell therapy offers targeted treatment for recurrent cervical cancer
Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543-1550.
Stevanovic and colleagues have been developing another immune-based therapy that has been tested for other cancers. This uses a method for generating T-cell cultures from HPV-positive cancers and selecting specific HPV oncoprotein- reactive cultures for administration to patients. Termed adoptive T-cell therapy (ACT), this targeted approach to recurrent cervical cancer is what I would consider one of the most intriguing future treatments of cervical disease. In the past, the largest barrier to an effective HPV vaccine to treat cervical cancer has been lack of clinical response to existing cytotoxic regimens. In this, albeit small, trial, investigators found a correlation between HPV reactivity and the infused T cells and objective clinical responses.
What is adoptive T-cell therapy?
ACT allows for more rigorous control over the magnitude of the targeted response than tumor vaccination treatment strategies because the T cells used for therapy are identified and selected in vitro. The cells selected are exposed to cytokines and immunomodulators that influence differentiation during priming and are expanded to large numbers. The resulting number of antigen-specific T cells produced in the peripheral blood is much greater (more than 10-fold) than that possible by current vaccine regimens alone.
Studies conducted by the National Cancer Institute of adoptive transfer of in vitro-selected tumor-infiltrating lymphocytes were the first to demonstrate the potential of T-cell immunotherapy to eradicate solid tumors.5,6 Among 13 patients with melanoma, treatment with adoptive transfer of ex vivo-amplified autologous tumor-infiltrating T cells resulted in treatment response in 10 of the patients—clinical responses in 6 and mixed responses in 4.
Details of the study
This study by Stevanovic and colleagues involved 9 patients with metastatic cervical cancer who previously had received optimal recommended chemotherapy or concomitant chemoradiotherapy regimens. Patients were treated with a single infusion of tumor-infiltrating T cells specifically selected for HPV E6 and E7 reactivity (HPV-TILs). Patients received lymphocyte-depleting chemotherapy before ACT and aldesleukin chemotherapy injection after ACT.
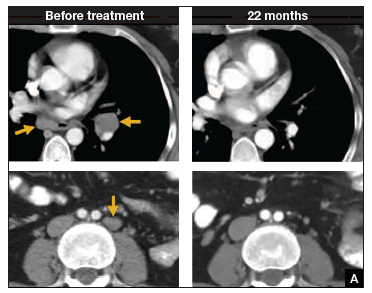
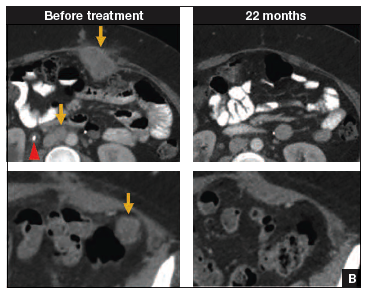
In such a phase I population, one would not expect clinical responses over persistent stable disease. However, in this small trial, 2 patients had complete tumor regression and 1 patient had a partial treatment response, demonstrating that a complete response to metastatic cervical cancer can occur after a single infusion of HPV-TILs. The partial response lasted 3 months. The 2 complete responses were ongoing 22 and 15 months after treatment (FIGURE 2).
Editorialists point out that, only when the infusion product had reactivity against the HPV E6 and E7 peptides did the patients show objective clinical response, suggesting it was the immune response that contributed to the tumor regression.7 In addition, in the 3 patients with objective responses, HPV-specific T cells persisted in peripheral blood for several months.
| FIGURE 2 Patients with complete tumor responses with adoptive T-cell therapy | ||
| 
| |
Two patients with metastatic cervical cancer had complete tumor responses with treatment with tumor-infiltrating T cells selected for HPV E6 and E7 reactivity (HPV-TILs). Contrast-enhanced computed tomography scans obtained before treatment and at most recent follow-up for both patients. (A) First patient (patient 3) had disease involving para-aortic, bilateral hilar, subcarinal, and left iliac lymph nodes (gold arrows). Patient had no evidence of disease 22 months after treatment. (B) Second patient (patient 6) had metastatic disease in para-aortic lymph node, abdominal wall, aortocaval lymph node, left pericolic pelvic mass, and right ureteral nodule (gold arrows). Patient had no evidence of disease 15 months after treatment. (Red arrowhead indicates ureteral stent that was removed after right ureteral tumor regressed.) | ||
Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells.J Clin Oncol. 2015;33(14):1543–1550. Used with permission.
What this evidence means for practice
The recent approval of bevacizumab has been a major breakthrough in the treatment of advanced and recurrent cervical cancer. Although ACT is a treatment that is in early clinical development, it is the next major advance in this area. Its promise is currently limited, as the process is cumbersome and complex, involving surgical removal of a patient's lymph nodes, culturing of the T cells from the lymph nodes, and infusing the T cells with the oncoproteins that will train those T cells to infiltrate the cancer tumor. The process is wrought with potential problems in laboratory and translational techniques. However, this group of investigators from the NCI has perfected the process of ACT, creating T cells that will target the HPV that is integrated into each cervical cancer tumor.
The patients who demonstrated good T-cell reactivity against HPV were the ones who had a treatment response, which demonstrates the targeted precision of ACT therapy. There might come a day when we can select patients with recurrent cervical cancer who are going to have T-cell reactivity, and send them for treatment to a center specialized in ACT. Typically in phase 1 trials, we are happy to see a number of patients responding with stable disease. In this trial, 2 patients had a complete response. The results demonstrated by Stevanovic and colleagues are very exciting for the future treatment of patients with cervical cancer.







