Congressionally Directed Medical Research Programs Complement Other Sources of Biomedical Funding
Emphasized in CDMRP research opportunities are the specific needs of its advocacy communities. The CDMRP recognizes the value of firsthand experience with each of the targeted diseases, conditions, and injuries and has been a leader in integrating consumers (defined as a patient, survivor, family member, or caregiver of a person living with the disease, condition, or injury) into every aspect of a program’s execution. The value of consumer involvement is derived from each individual’s firsthand experience. This approach adds a perspective, passion, and sense of urgency, which ensures that the human dimension is incorporated in each program’s policy, investment strategy, and research focus. Consumers vote side by side with scientists and clinicians on advisory boards for each of the programs, and they have since the inception of the CDMRP.
Each research application must have an impact statement describing how the proposed research, if successful, will transform an aspect of the understanding, prevention, detection, and/or treatment of the respective program area; ie, have an impact on the consumer community. The impact of the proposed research is a critical determinant of the funding recommendation.
Each research program’s investment strategy and associated award mechanisms provide the framework and direction necessary to most effectively invest the congressional appropriation. Operationally, the CDMRP monitors for potentially similar approaches in research at many milestones in its science management model to ensure that the CDMRP-funded research is synergistic and harmonizing, not duplicative of other federal and nonfederal sources of funding.
At the time of proposal submission, a comprehensive list of current and pending funding support for the principal investigator (PI) and all key personnel must be submitted. During the review process, peer reviewers who have extensive knowledge of the subject consult the pending and existing support documentation to ensure the research is complementary to what is already being investigated in the field. This ensures that the proposals recommended for funding are synergistic and contribute to the substantiation of data relevant to clinical decisions. After a project has been recommended for funding, the CDMRP scientific officers (ie, scientific technical advisors) check all available sources to ensure that the project to be funded is complementary to ongoing research. Last, during the period of performance, details about funding applied for and/or new funding obtained is required in the annual technical progress reports. Through this science management model, CDMRP ensures that funded research is complementary and able to innovatively fill gaps in the biomedical research pipeline.
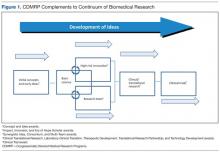
Biomedical Funding
Most diseases, conditions, and injuries are complex, and finding a cure for them requires problem solving from multiple disciplines and approaches as well as validation of research results. Prior to the fielding and clinical application of knowledge and products, research spans a continuum from discovery to clinical trials. As shown in Figure 1, novel award mechanisms developed by the CDMRP programs facilitate the success of this research continuum and innovatively complement traditional research funding agencies, such as the NIH. The intent of each award mechanism is designed to solicit research proposals focused on the needs of the patient community and how they relate to the vision of the program.
The Research Continuum
Some CDMRP programs provide support along the entire continuum of research. Other programs, with less mature research fields, focus on funding more basic research. There are also CDMRP programs that place emphasis on clinical and advanced development research. Each program’s annual investment strategy and choice of award mechanisms is based on the needs of the patient and research communities, gaps in research, and other barriers to progress in curing, rehabilitating, or eliminating the disease, condition, or injury.
Fostering the Development of Ideas
Since its inception in 1992, the DoD BCRP has sought to fund innovative, ground-breaking research by encouraging “outside the box” thinking and fostering creative collaborations that have the potential to have a high impact toward the eradication of this disease. The BCRP has a proven history of developing novel award mechanisms to foster new approaches in research. For example, the Idea Award was developed in the initial years of the BCRP to support novel research with little or no preliminary data that could ultimately lead to a critical discovery or advancement in breast cancer research. At that time, such high-risk, but potentially high-reward research was determined to be significantly underfunded by existing agencies and was thus identified as a gap in funding. Several major advancements in breast cancer, including the development of trastuzumab, testing of sentinel lymph node biopsy, and discovery of BRCA2 and PTEN gene mutations, were supported in part with funding from the BCRP.