Increasing Local Productivity Through a Regional Antimicrobial Stewardship Collaborative
Background: Antimicrobial stewardship programs (ASPs) are vital to improving patient safety and ensuring quality of care but are often underresourced, limiting their effectiveness and reach. While barriers to ASP success have been well documented, approaches to address these barriers with limited resources are needed. Stewardship networks and collaboratives have emerged as possible solutions. In January 2020, 5 US Department of Veterans Affairs facilities created a regional ASP collaborative. In this article, we describe the impact of this collaborative on the productivity of the facilities’ ASPs.
Methods: ASP annual reports for each of the 5 facilities provided retrospective data. Reports from fiscal year (FY) 2019 and reports from FY 2020-2022 were reviewed. Staffing, inpatient and outpatient stewardship reporting, individual and collaborative initiatives, and publications data were collected to measure productivity. Yearly results were trended for each facility and for the region. Additionally, the COVID-19 antibiotic use dashboard and upper respiratory infection dashboard were used to review the impact of initiatives on antibiotic prescribing during the collaborative.
Results: Regular reporting of outpatient metrics increased; 27% of measures showed improvement in 2019 and increased to 60% in 2022. For all 5 facilities, ASP initiatives increased from 33 in 2019 to 41 in 2022 (24% increase) with a corresponding increase in collaborative initiatives from 0 to 6. Likewise, publications increased from 2 in 2019 to 17 in 2022 (750% increase). Rates of reporting and improvement in inpatient metrics did not change significantly.
Conclusions: The ASP collaborative aided in efficiency and productivity within the region by sharing improvement practices, distributing workload for initiatives, and increasing publications.
Results
In 2019, only 2 sites (A and C) reported dedicated FTE compared with recommended minimum staffing; neither met minimum requirements. In 2020, 1 facility (B) met the physician FTE recommendation, and 2 facilities met the pharmacy minimum FTE (D and E). In 2021 and 2022, 2 of 5 facilities (B and E) met the physician minimum FTE, and 2 of 5 (D and E) met the minimum pharmacy FTE recommendations. For the study years 2019 to 2022, 1 facility (E) met both pharmacy and physician FTE recommendations in 2021 and 2022, and 2 facilities (A and C) never met minimum FTE recommendations.
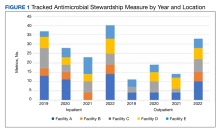
Regarding ASP metrics, all facilities tracked and reported inpatient AU; however, facility A did not document inpatient metrics for FY 2021. The number of individual inpatient metrics varied annually; however, FY 2022 saw the highest reported for the VISN (n = 40), with a more even distribution across facilities (Figure 1). Common metrics in 2022 included total AU, broad-spectrum gram-negative AU, anti–methicillin-resistant Staphylococcus aureus (MRSA) agent use, antibiotics with high risk for Clostridioides difficile infection (CDI), and AU in patients with COVID-19. The percentage of improved metrics for VISN 9 was consistent, ranging from 26.5% to 34.8%, throughout the study period.
From 2019 to 2022, facilities reporting outpatient AU increased from 3 to 5 and included fluoroquinolone use and AU in ARI. VISN 9 outpatient metrics increased every year except in 2021 with improved distribution across facilities. The number of total metrics with reported improvement in the outpatient setting overall increased from 3 of 11 (27%) in 2019 to 20 of 33 (60%) in 2022.
Antimicrobial Stewardship Initiatives
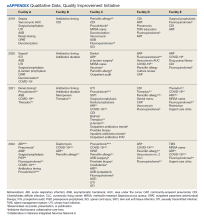
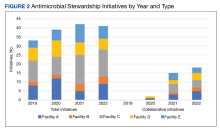
Quantitative and qualitative data regarding initiatives are reported in Figure 2 and the eAppendix respectively. Since the formation of the collaborative, total initiatives increased from 33 in 2019 to 41 in 2022. In 2019, before the collaborative, individual facilities were working on similar projects in parallel, which included MRSA decolonization (A and C), surgical prophylaxis (A and E), asymptomatic bacteriuria (A and C), and CDI (B, C, D, and E). The development of clinical pathways and order sets remained consistent, ranging from 15 to 19 throughout the study period except for 2020, when 33 clinical pathways and/or order sets were developed. Collaboration between sites also remained consistent, with 1 shared clinical pathway and/or order menu between at least 1 site reported yearly for 2020, 2021, and 2022. The number of publications from VISN 9 grew from 2 in 2019 to 17 in 2022. In 2019, there were no collaborative research or QI publications, but in 2022 there were 2 joint publications, 1 between 2 facilities (A and C) and 1 including all facilities.
ARI and COVID-19 were identified by the collaborative as VISN priorities, leading to shared metrics and benchmarking across facilities. From 2019 to 2022, increased collaboration on these initiatives was noted at all facilities. The ARI goal was established to reduce inappropriate prescribing for ARI/bronchitis to under 20% across VISN 9. Rates dropped from 50.3% (range, 35.4%-77.6%) in FY 2019 quarter (Q) 1 to 15% (range, 8%-18.3%) in FY 2022 Q4. The clinical guidance subcommittee developed a guideline for AU in patients with COVID-19 that was approved by the VISN 9 Pharmacy & Therapeutics Committee. A VISN 9 dashboard was developed to track inpatient and outpatient AU for COVID-19. Antibiotic prescribing in the first 4 days of hospitalization decreased from 62.2% at the start of the COVID-19 pandemic to 48.7% after dissemination of COVID-19 guidance.