Impact of Liraglutide to Semaglutide Conversion on Glycemic Control and Cost Savings at a Veterans Affairs Medical Center
Background: Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) approved by the US Food and Drug Administration for patients with type II diabetes mellitus (T2DM). Patients with T2DM treated with liraglutide at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) were converted to semaglutide. The primary objective was to assess changes in glycemic control and cost savings that resulted from this conversion.
Methods: We conducted a retrospective chart review of veterans without retinopathy treated at MEDVAMC between March 1, 2021, and November 30, 2021, who were converted from liraglutide 0.6 mg and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks). To assess glycemic control, we compared hemoglobin A1c (HbA1c) values 3 to 12 months following conversion with baseline values. Cost savings were evaluated using outpatient pharmacy data.
Results: During the study, 411 patients were converted from liraglutide to semaglutide; 49 additional patients met the criteria for clinician education, and 14 were converted as a result. In total, 304 patients met the criteria for inclusion. At baseline, patients’ levels included: mean (SD) HbA1c, 8.1% (1.5); blood glucose, 187 (44.2) mg/dL; and body weight, 112.9 (23) kg. Three to 12 months postconversion, patients’ mean (SD) HbA1c significantly decreased to 7.6% (1.4) (P < .001), blood glucose decreased to 172.6 (39) mg/dL (P < .001), and body weight decreased to 105.2 (32.3) kg (P < .001). Cost savings exceeding $400,000 resulted from the conversion from liraglutide to semaglutide.
Conclusions: Conversion of liraglutide to semaglutide led to significant HbA1c decrease and weight loss and resulted in minimal changes to patients’ antihyperglycemic regimen. Common adverse effects included hypoglycemia and gastrointestinal intolerance. Due to the low conversion rate of liraglutide to semaglutide following education, a more effective method of education for clinicians to promote teleretinal imaging before conversion is warranted. Lastly, although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings for the institution, a full cost-effective analysis is needed for further conclusion.
Results
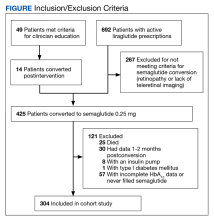
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
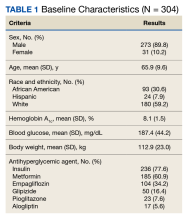
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
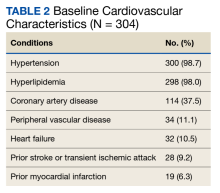
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
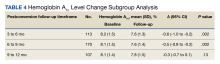
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
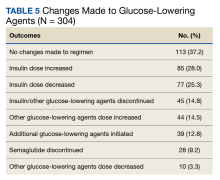
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.