The Critical Value of Telepathology in the COVID-19 Era
Background: Telepathology, which includes the use of telecommunication links, helps enable transmission of digital pathology images for primary diagnosis, quality assurance, education, research, or second opinion diagnoses.
Observations: This review covers all aspects of telepathology implementation, including the selection of platforms, budgets and regulations, validation, implementation, education, quality monitoring, and the potential to improve practice. Considering the long-term trends, the lessons of the COVID-19 pandemic, and the potential for future pandemics or other disasters, the validation and implementation of telepathology remains a reasonable choice for laboratories looking to improve their practice.
Conclusions: Though barriers to implementation exist, there are potential benefits, such as the wide spectrum of uses like frozen section, telecytology, primary diagnosis, and second opinions. Telepathology represents an innovation that may transform the future of pathology practice.
Validation of telepathology by the testing site demonstrates that the new system performs as expected for its intended clinical use before being put into service and that the digital slides produced are acceptable for clinical diagnostic interpretation.11 The College of American Pathologists (CAP) established WSI validation guidelines are part of the published laboratory standard of care.11-13 An appropriate validation enables the benefits of telepathology while mitigating the risks.
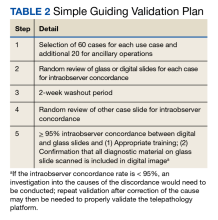
There are 3 major CAP recommendations for validation. First, ≥ 60 cases should be included for each use case being validated with 20 additional cases for relevant ancillary applications not included in the 60 cases. Second, diagnostic concordance (ideally ≥ 95%) should be established between digital and glass slides for the same observer. Third, there should be a 2-week washout period between the viewing of digital and glass slides (Table 2).12,13
Guidelines from the ATA establish that telepathology systems should be validated for clinical use, including non-WSI platforms.2 Published validations of other non-WSI platforms (such as by robotic or multimodality telepathology) have followed the structure proposed in the guidelines by CAP for validating WSI.14,15
Ensuring that all relevant responsibilities (clinical, facility, technical, training, documentation/archiving, quality management, and operations related) for the use of telepathology are met is another aspect of validation and implementation.2 Clinical responsibilities include an agreement between the sending (referring) and receiving (consulting) parties on the information to accompany the digital material.2 From ATA clinical guidelines, this includes identification information, provision to the consulting pathologist of all relevant clinical data, provision to retrieve for access any needed and/or relevant diagnostic material, and responsibility by referrer that the correct image/metadata was sent.2 Involved parties should be trained to manage the materials being transmitted.2
Facility responsibilities include maintaining the standard of care defined by the facility and regulatory agencies.2 The maintenance of accreditation, adherence to licensure requirements, and proper management of privileges to practice telepathology are also important.2 Technical responsibilities include ensuring a proper validation that meets the standard of care and covers use cases.2,11-13
All processes, training, and competencies should be followed and documented per standard facility operating procedures.2 ATA recommends that telepathology should result in a formal report for diagnostic consultations, maintain logs of telepathology interactions or disclaimer statements, and have an appropriate retention policy.2 The CAP recommends digital images used for primary diagnosis should be kept for 10 years if the original glass slides are not available.16 Once implemented, telepathology reports must be incorporated into the pathology and laboratory medicine department’s quality management plan for both the technical performance of the telepathology system and diagnostic performance of the pathologists using the system.2 Operations responsibilities include ensuring that the telepathology system is maintained according to vendor recommendations and regulatory standards. Appropriate provisions for space and associated needs should be developed in conjunction with the information technology team of the facility to ensure appropriate security, privacy, and regulatory compliance.2