Pharmacist-Led Management of HIV PrEP Within the Veterans Health Administration
Background: Uptake and access to HIV preexposure prophylaxis (PrEP) is key to reducing incident HIV infections. Pharmacists are one of the most accessible health care professionals in the United States and are well suited to address this need.
Observations: We describe a model of care at the Veterans Affairs Greater Los Angeles Healthcare System in which clinical pharmacist practitioners developed and implemented a pharmacy-led PrEP clinic colocated within an infectious disease clinic. Veterans Health Administration clinical pharmacists provide direct patient care under a scope of practice that includes ordering and interpreting laboratory tests and providing PrEP prescriptions. To improve access and patient acceptability, we also used novel telemedicine modes of care to ensure flexible appointment scheduling.
Conclusions: This model can be used by other federal and community-based health care organizations to implement interdisciplinary pharmacist-managed PrEP clinics and expand telehealth modalities to deliver outpatient services.
Clinic Quality of Care
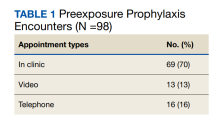
From July 2019 to March 2020, 53 veterans were managed by the pharm-PrEP clinic in 98 encounters. Seventy percent of encounters were in-person (Table 1).
Baseline information collected included demographics, documented patient-reported risk factors, fourth-generation HIV screening test results, STI status, viral hepatitis serologies, and renal function test results. Information collected every 3 to 6 months included STI status, fourth-generation HIV screening test results, renal function test results, adherence to therapy, changes in risk factors, and prescription refill data. Additional interventions conducted as part of clinic workflow included DEXA scans, vaccinations, and active prescriptions for condoms.
Baseline Characteristics
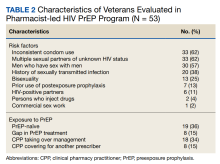
Pharm-PrEP clinic patients were predominantly male (94%), and a majority indicated White race with a median age of 38 years (range, 24-80 years).
Veterans referred to the clinic had up to 5 risk factors for PrEP initiation. The most common risk factors were inconsistent condom use (62%), multiple sexual partners of unknown HIV status (62%), MSM (57%), STI history (38%), bisexual partners (25%), and HIV-positive sexual partners (11%). One of the 53 individuals referred for PrEP had no risk factors and did not initiate PrEP. Two individuals declined initiation of PrEP after consultation. Twenty six of 53 veterans at baseline continued their use of PrEP following transfer to clinic CPP management; 24 of 27 veterans not currently using PrEP (89%) started or restarted lapsed PrEP use following CPP consultation.
HIV and STI Screening
No individuals tested positive for HIV at baseline (n = 52) or while on PrEP. PrEP was not renewed for 3 patients that did follow through with HIV testing. The median number of days an HIV test was completed prior to initial PrEP and PrEP renewal was 4 days and < 7 days, respectively, both of which are below the recommended maximal interval of 7 days, according to CDC PrEP guidelines. Some postinitiation HIV testing occurred using a longer interval of 14 days, in accordance with VA National Criteria for Use of PrEP. This modification allowed more flexibility as a majority of PrEP prescriptions are sent to veterans via mail. The CPP reviewing HIV test results was able to expedite the processing and mailing of PrEP prescriptions if deemed appropriate, ie, the HIV test was negative. This approach was not used if a patient had high-risk exposures without PrEP during the time between collection of the HIV test and mailing of the prescription.