Randomized, Double-Blind Placebo-Controlled Trial to Assess the Effect of Probiotics on Irritable Bowel Syndrome in Veterans With Gulf War Illness
Background: Many veterans who served in Operation Desert Storm (August 1990 to March 1991) experienced a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly impacts the health and quality of life (QOL) and may have contributed to irritable bowel syndrome (IBS).
Methods: We performed a prospective, double-blind placebo-controlled study to determine the efficacy of the multistrain De Simone Formulation probiotic containing 8 strains of bacteria on symptoms of IBS and GWI. Veterans of Operation Desert Storm who had IBS and ≥ 2 nonintestinal symptoms of GWI were included. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, and extra-intestinal and posttraumatic stress disorder (PTSD) symptoms.
Results: A total of 101 Gulf War veterans with IBS and GWI were screened at the Veteran Affairs Medical Center in Salt Lake City, Utah. The study was completed by 53 veterans; 47 (89%) were male with a mean (SD) age of 55 (8) years. The probiotic did not improve IBS symptoms or other extra-intestinal symptoms common to IBS and GWI.
Conclusions: Our study did not demonstrate statistically significant improvement in IBS symptoms or QOL after treatment with the probiotic. We also did not find any improvement in symptoms of GWI or PTSD.
Results
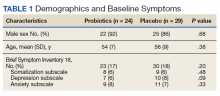
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
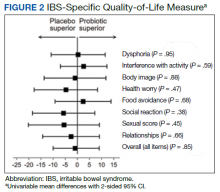
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.