Pharmacist-Assisted Varenicline Tobacco Cessation Treatment for Veterans
Background: Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. The purpose of this study was to evaluate the efficacy and safety of management of varenicline by clinical pharmacy specialists (CPSs) compared with other clinicians.
Methods: This retrospective chart review included patients with a varenicline prescription between July 1, 2019, and July 31, 2020. Primary outcomes were reduction in tobacco use at 12 weeks from baseline, continuous abstinence at 12 weeks, adherence to varenicline therapy, and time to first follow-up. For safety evaluation, charts were reviewed for documented adverse drug reactions.Results: Management by CPS compared with other clinicians was associated with similar mean (SD) reductions of tobacco use (-7.9 [10.4] vs -5.4 [9.8] cigarettes per day, respectively; P = .15) and rates of complete abstinence (34% vs 38%, respectively; P = .73) and higher adherence (42% vs 31%, respectively; P = .01). Mean (SD) time to first follow-up was shorter for patients in the CPS group: 52 (66) vs 163 (110) days; P < .001. Adverse events were more common in the CPS group compared with the other clinicians group (42% vs 23%; P = .02).
Conclusions: These results suggest that CPS management of varenicline is as safe and effective as management by other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of CPS scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
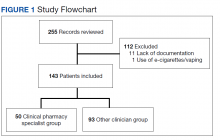
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
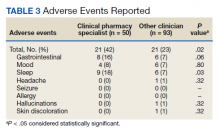
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
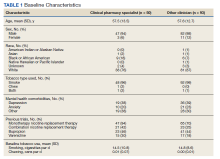
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
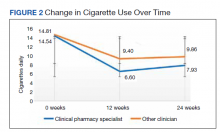
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.