Natriuretic Peptide Screening for Primary Prevention or Early Detection of Heart Failure: A Pharmacist-Driven Team-Based Approach
Background: Emerging data indicates that natriuretic peptide biomarker-based screening and early intervention could prevent left ventricular dysfunction or new-onset heart failure (HF). The 2017 update of the American College of Cardiology/American Heart Association/Heart Failure Society of America guideline for managing HF provides a IIa recommendation for natriuretic peptide biomarker screening followed by a team-based approach for preventing HF.
Observations: Clinical pharmacists worked collaboratively with a cardiology specialist and primary care practitioners to establish a protocol to identify patients at risk for HF. Patients with hypertension and/or type 2 diabetes mellitus (T2DM) and without a history of HF with N-terminal pro-B-type natriuretic peptide > 125 pg/mL received follow-up from clinical pharmacists, including initiation and/or adjustment of renin-angiotensin system inhibitors, discussion of echocardiogram, and comprehensive disease state management of hypertension, T2DM, atherosclerotic cardiovascular disease risk reduction, oral nonsteroidal anti-inflammatory drug reduction, and tobacco cessation.
Conclusions: By using natriuretic peptide screening, clinical pharmacists were able to identify patients with hypertension and/or T2DM who were at higher risk for HF and provide comprehensive medication management.
Patient Selection
Patients aged ≥ 18 years with hypertension, taking antihypertensive medication for ≥ 1 month, or diagnosed with T2DM for ≥ 6 months were included. Using the parameters provided in the STOP-HF trial, patients with evidence or history of left ventricular dysfunction, defined as a left ventricular ejection fraction (EF) < 50% or an E/e’ ratio > 15 in the setting of normal EF, or symptomatic HF were excluded. Patients with a diagnosis causing life expectancy < 1 year were excluded, which was determined based on review of the patient’s chart or discussion with the PCP.
A clinical pharmacist screened patients with an upcoming PCP appointment between September 2019 and January 2020 for eligibility. For patients who met criteria, the clinical pharmacist ordered a NT-proBNP laboratory test to their already scheduled tests and entered a templated note into the patient’s chart to alert the PCP of the test. NT-proBNP was used rather than BNP because it was the natriuretic peptide laboratory test available at CJZVAMC during this time. Patients with NT-proBNP < 125 pg/mL received usual care from their PCPs. Patients with NT-proBNP ≥ 125 pg/mL received a follow-up phone call from a clinical pharmacist to discuss the laboratory test result with recommendations for initiation or increase of RAS inhibitors and an echocardiogram. If the patient agreed to an echocardiogram, the PCP was notified to order the test. For patients aged > 80 years with elevated NT-proBNP, risk vs benefit and patient-specific goals of care were discussed with the PCP. For patients whose echocardiograms revealed left ventricular dysfunction, initiation or adjustment of β blockers was considered. During RAS inhibitor increase, the clinical pharmacists provided a review of the patient’s risk factors and optimized management of hypertension, T2DM, ASCVD risk reduction, oral nonsteroidal anti-inflammatory drug (NSAID) reduction, and tobacco cessation.
Outcome Measures
Outcome measures included the percentage of patients who met inclusion/exclusion criteria and had an elevated NT-proBNP level, percent change in RAS inhibitor prescriptions and optimized dosing after intervention, frequency of left ventricular dysfunction visualized with echocardiograms, and quantification of pharmacist interventions in disease state management. Descriptive statistics were used to analyze demographic data, RAS inhibitors prescriptions before and after intervention, echocardiogram results, pharmacist recommendations, and acceptance rates of disease state management.
Results
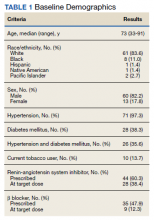
Between September 2019 and January 2020, 570 patients from 2 PACT teams were screened. Of the 570 patients, 246 met inclusion criteria with upcoming appointments. Of these, 24 were excluded, 10 for EF < 50%, 13 for E/e’ > 15 in setting of normal EF, and 1 for hypertension diagnosis without an antihypertensive regimen or elevated blood pressure. The remaining 222 patients had an NT-proBNP level ordered and drawn and 73 (32.9%) patients had an NT-proBNP ≥ 125 pg/mL. Baseline characteristics are described in Table 1.
Data was collected through March 2020 (due to COVID-19) found that among the 73 patients with elevated NT-proBNP: 14 had an echocardiogram within the past year without evidence of left ventricular dysfunction; 39 had echocardiograms ordered; and 19 had echocardiograms completed by March 2020. Among the 19 echocardiograms, 16 (84%) showed no evidence of left ventricular dysfunction, 2 (11%) revealed mildly reduced EF (40% to 50%), and 1 (5%) revealed a reduced EF (< 40%). These patients were identified early in the disease course before symptom onset and received intervention with RAS inhibitors and disease state management.
Patients prescribed RAS inhibitors increased from 44 to 50. The number of patients who were able to have their RAS inhibitor dosage adjusted increased from 28 to 31. For the 3 patients with mildly reduced or reduced EF, management with β blockers was based on RAS inhibitor adjustment toleration. One patient with mildly reduced EF was switched from metoprolol tartrate to metoprolol succinate.
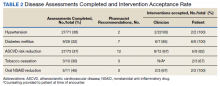
Clinical pharmacists completed disease state assessments to optimize management of hypertension, T2DM, ASCVD risk reduction, oral NSAID reduction, and tobacco cessation (Table 2). Interventions clinical pharmacists recommended for hypertension, in addition to RAS inhibitor management, included initiation and adjustment of amlodipine. For T2DM, interventions included initiation of metformin and initiation or adjustment of empagliflozin. For ASCVD risk reduction, interventions included starting a statin or adjusting statin therapies to appropriate intensities based on clinical ASCVD 10-year risk. Tobacco cessation interventions included pharmacotherapies, counseling, and education with written materials. Pharmacists counseled patients to minimize or eliminate NSAID use and, when appropriate, discontinued active oral NSAID prescriptions.
Discussion
We included patients diagnosed with T2DM and hypertension for several reasons. Most patients (62%) studied in the STOP-HF trial were diagnosed with hypertension. Also, T2DM represented the patient population enrolled in the PONTIAC trial. Guidance from the European Society of Cardiology recommends use of natriuretic peptides in high-risk populations, such as patients with DM and hypertension, to help target initiation of preventive measures.7 Lastly, T2DM and hypertension patients were easily identified using population management software available at the VA.