Assessing Outcomes Between Risperidone Microspheres and Paliperidone Palmitate Long-Acting Injectable Antipsychotics Among Veterans
Background: Long-acting injectable antipsychotics (LAIAs) are integral for managing schizophrenia, schizoaffective disorder, and bipolar I disorder among veterans. Studies comparing LAIAs, such as risperidone microspheres and paliperidone palmitate, are limited. The primary objective of our study was to compare the number of psychiatric hospitalizations among veterans initiated on risperidone microspheres and those taking paliperidone palmitate pre- and post-LAIA initiation.
Methods: We included veterans who had received ≥ 2 injections of risperidone microspheres or paliperidone palmitate between January 1, 2016 and December 31, 2018. Nonadherence was defined as missing an injection by > 3 days for risperidone microspheres and > 7 days for paliperidone palmitate. Pre-LAIA and post-LAIA hospitalizations and rehospitalizations were assessed using a pre–post design with equivalent periods. Descriptive statistics were used for demographics and diagnoses. Nonparametric tests were used to analyze primary and secondary outcomes.
Results: The study included 97 veterans; 44 took risperidone microspheres and 53 received paliperidone palmitate. Participants’ average mean (SD) age was 46 (13.8) years, 92% were male, and 94% were diagnosed with schizophrenia or schizoaffective disorder. Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02), were less likely to be rehospitalized (22.7% vs 47.2%; P = .013) and had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04) compared with paliperidone palmitate.
Conclusion: Veterans receiving risperidone microspheres had fewer posttreatment psychiatric hospitalizations and were less likely to be rehospitalized .
Results
We screened 111 veterans, and 97 were included in this study (risperidone microspheres, 44; paliperidone palmitate, 53). Mean (SD) age was 46 (13.8) years, 92% were male, 38% were White, 94% were diagnosed with schizophrenia or schizoaffective disorder, and 11% were homeless. Substance use was documented as 52% for nicotine products, 40% for alcohol, 31% for cannabis, 27% for methamphetamine, 7% for cocaine, and 3% for opioids. Cannabis, methamphetamine, cocaine, and opioid use were based on clinician documentation and listed as active diagnoses at the time of LAIA initiation. Statistical significance was found in index hospitalizations P = .009) and history of cocaine use disorder (6.8% vs 7.5%, P < .001).
Veterans administered risperidone microspheres had fewer mean (SD) post-LAIA hospitalizations (0.4 [1.0] vs 0.9 [1.5]; P = .02) and were less likely to be rehospitalized (22.7% vs 47.2%, P = .01) compared with paliperidone palmitate. However, veterans taking risperidone microspheres had a shorter mean (SD) treatment duration (41.6 [40.2] vs 58.2 [45.7] weeks, P = .04) compared with paliperidone palmitate, mainly because patients switched to a different LAIA or oral antipsychotic. No differences were detected in nonadherence and discontinuation between risperidone microspheres and paliperidone palmitate. All veterans in the risperidone microspheres group adhered to oral risperidone crossover with an average 87.8% days covered (Table 2).
The average maintenance dose of risperidone microspheres was 42 mg every 2 weeks and 153 mg every 4 weeks for paliperidone palmitate.
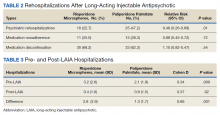
Across the sample, 84% of veterans had a previous psychiatric hospitalization, although veterans initiated on risperidone microspheres had significantly higher mean (SD) index hospitalizations than those started on paliperidone palmitate (3.2 [2.6] risperidone microspheres vs 2.1 [1.9] paliperidone palmitate, P = .009). Both groups had significant decreases in mean (SD) hospitalizations (3.2 [2.6] to 0.4 [1.0], risperidone microspheres vs 2.1 [1.9] to 0.9 [1.5] paliperidone palmitate). The risperidone microspheres group had a larger decrease in mean (SD) hospitalizations post-LAIA (2.8 [2.9] risperidone microspheres vs 1.3 [1.7] paliperidone palmitate, P = .001) (Table 3).
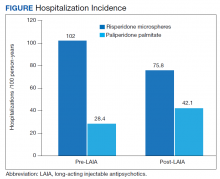
Differences in incidence per 100 person-years between pre- and post-LAIA hospitalizations were larger in risperidone microspheres users than in paliperidone palmitate (73.8 vs 33.7, P = .01) (Figure). No differences between risperidone microspheres and paliperidone palmitate were detected when looking at incidence pre-LAIA (102.2 vs 75.8, P = .22) and post-LAIA (28.4 vs 42.1, P = .38) separately.
Thirty veterans in the risperidone microspheres group discontinued LAIA: 11 were nonadherent, 5 experienced adverse effects (AEs), and 14 discontinued due to inconvenience. Among 33 veterans in the paliperidone palmitate group who discontinued the LAIA, 15 were nonadherent, 11 experienced AEs, 4 stopped due to of inconvenience, and 3 switched to a less frequently administered LAIA. The most common AEs reported were injection site reactions, cholinergic AEs (salivation, lacrimation, urination), orthostasis, and weight gain.
Discussion
The main finding of this study was that initiation of LAIAs significantly reduced hospitalizations. Veterans taking risperidone microspheres had higher index hospitalizations and lower posttreatment hospitalizations compared with paliperidone palmitate. We found that patients initiated on risperidone microspheres had more hospitalizations before use of a LAIA than those initiated on paliperidone palmitate. Risperidone microspheres reduced the number of hospitalization post-LAIA significantly more than paliperidone palmitate. We also found that veterans taking risperidone microspheres were on the medication for less mean (SD) time than those on paliperidone palmitate (41.6 [40.2] vs 58.2 [45.7] weeks; P = .04).
To our knowledge, this is one of the few studies that compared outcomes of psychiatric hospitalizations, medication adherence, and treatment discontinuation between risperidone microspheres and paliperidone palmitate, specifically in a veteran population.16-19 Limosin and colleagues aimed to compare length of stay during the initial hospitalization, rehospitalization risk, and treatment duration between risperidone microspheres and paliperidone palmitate in patients with schizophrenia.16 These researchers detected no differences in initial hospitalization duration and time to rehospitalization between risperidone microspheres and paliperidone palmitate.16 The study revealed a more favorable trend in time to discontinuation for paliperidone palmitate, but switching between LAIAs might have confounded the data.16 The authors note that their study lacked power, and patients on paliperidone palmitate had significantly more nonpsychiatric comorbidities.16 Joshi and colleagues looked at adherence, medication discontinuation, hospitalization rates, emergency department visits, and hospitalization costs between risperidone microspheres and paliperidone palmitate in patients identified in Truven MarketScan Commercial, Medicare Supplemental, and Medicaid Multi-State insurance databases.17 The authors found paliperidone palmitate to be superior in all objectives with better adherence, lower discontinuation rates, less likelihood of hospitalization, fewer emergency department visits, and lower hospitalization costs compared with risperidone microspheres.17 Korell and colleagues aimed to establish reference ranges for plasma concentrations of risperidone and paliperidone among adherent patients.18