Implementation and Impact of a β -Lactam Allergy Assessment Protocol in a Veteran Population
Background: Approximately 10% of the US population reports having a β -lactam allergy, although nearly 90% do not have a true immunoglobulin E (IgE)-mediated reaction. This misconception results in using nonpreferred antibiotics, leading to antimicrobial resistance and treatment failure. To evaluate, clarify, and clear β -lactam allergies, we implemented a pharmacist-driven β -lactam allergy assessment (BLAA) protocol and penicillin allergy clinic (PAC). The purpose of this study was to illustrate the BLAA process, including the pharmacist-run PAC, and assess the impact on allergy clearance.
Methods: Clinical pharmacy specialists (CPS) evaluated hospitalized veterans with β -lactam allergies, using the BLAA protocol. Eligible patients could later be seen in PAC. This was a retrospective observational review of the BLAA protocol to assess recommendations for β -lactam antibiotic use and PAC outcomes.
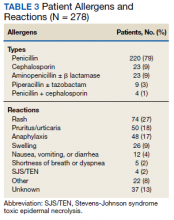
Results: Between November 2017 and February 2020, 278 patients were evaluated, and 32 were seen in the clinic. The most common allergen was penicillin, and the most reported reaction was a rash (27%) or pruritus and urticaria (18%). Through PAC and the BLAA protocol, 86 patients (31%) were cleared for allergy removal, and 188 (68%) were cleared for alternative β -lactams. The evaluation revealed that 274 patients (99%) were eligible to receive a β -lactam antibiotic, and only 4 patients (1%) were recommended for avoidance of all β -lactams.
Conclusions: These findings highlight the utility of the pharmacist-driven BLAA protocol. We illustrated that most patients with documented β -lactam allergies were eligible for alternative β -lactams. The implementation of the BLAA protocol and pharmacist-run PAC facilitated allergy clearance and has the potential to promote alternative β -lactam use.
Results
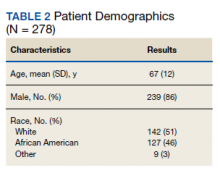
We evaluated 278 patients, using the BLAA protocol. In this veteran population, patients were generally older males and evenly split between African American and White patients (Table 2). Most patients reported an allergy to penicillin, with a rash being the most common reaction (Table 3).
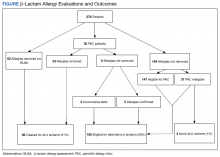
Of the 278 assessed, 246 patients were evaluated via our BLAA alone and were not seen in PAC. We were able to remove 25% of these patients’ allergies by performing a thorough assessment. Of the 184 patients whose allergies could not be removed via the BLAA alone, 147 (80%) were still eligible for PAC but are awaiting scheduling. Patients ineligible for PAC included those with a cephalosporin allergy or a severe and non–IgE-mediated reaction. Other ineligible patients who were not eligible included those with diseases where risk of testing outweighed the benefits.
Of the 32 patients who were seen in PAC, 75% of allergies were removed through direct testing. No differences between race or gender were observed. Of the 8 patients (25%) whose allergies were not removed, 5 had confirmed penicillin allergies with a positive reaction; 4 of these patients have since tolerated an alternative β-lactam (either a cephalosporin or carbapenem). Three patients had inconclusive tests, most often because their positive control was nonreactive during the percutaneous portion of the skin test; these allergies could neither be confirmed nor removed. Two of these patients have since tolerated alternative β-lactams (both cephalosporins). Although these 8 patients should not be rechallenged with a penicillin antibiotic, they could still be considered for alternative β-lactams, based on the nature and histories of their allergies.
In total, we removed 86 allergies (31% of our patient population) using both BLAA and PAC (Figure). These patients were cleared for all β-lactams. One hundred eighty-eight patients (68%) were cleared to receive an alternative β-lactam based on the nature or history of the allergic reaction. β-lactam avoidance was recommended for only 4 patients (1%), as they had no exposure to any β-lactams, and they had a recent or severe reaction: 2 patients with anaphylaxis in the past 5 years, 1 with SJS/TEN, and 1 with recent convulsions after receiving cefepime. Combining patients whose penicillin allergies were removed with those who had been cleared for alternative β-lactam antibiotics, 99% of patients were cleared for a β-lactam antibiotic.
Discussion
We have implemented a unique and efficient way to evaluate, clarify, and clear β-lactam allergies. Our BLAA protocol allows for a smooth process by distributing the workload of evaluating and clarifying patients’ allergies over many inpatient CPS. Furthermore, the BLAA is readily accessible to health care providers (HCPs), allowing for optimal clinical decision making. HCPs can quickly gather further information on the β-lactam allergy, while seeing actionable recommendations, along with documentation of the PAC visit and subsequent events, if the patient has been seen.
This study demonstrated the promotion of alternative β-lactam use for nearly all patients: 99% of our patient population were deemed candidates for a β-lactam type antibiotic. This percentage included patients whose allergies have been fully cleared, both through BLAA alone and in PAC. Also included are patients who have been cleared for an alternative β-lactam and not necessarily a penicillin.
In our PAC, 8 patients were not cleared for penicillins: 5 had penicillin allergies confirmed, and 3 had inconclusive results. Based on the nature of their reactions and previous tolerance of alternative β-lactams, those 5 patients are still eligible for alternative β-lactams. Additionally, the 3 patients with inconclusive results are also eligible for alternative β-lactams for the same reasons. The patients for whom