Real-World Experience With Automated Insulin Pump Technology in Veterans With Type 1 Diabetes
Background: Advancements in diabetes technology now allow insulin pump and continuous glucose monitor (CGM) technology to be a part of usual US Department Veterans Affairs (VA) clinical care. The automated insulin pump (AIP) delivers insulin automatically based on CGM readings. In randomized clinical trials the closed-loop system has shown to improve glycemic control in children and younger adults with type 1 diabetes mellitus (T1DM) while preventing hypoglycemia. However, its safety and efficacy is less well known in older veterans with T1DM. In this VA pilot study, we aimed to assess AIP technology in the real world of an older population of veterans with T1DM followed in the outpatient setting.
Methods: Thirty-seven patients with T1DM new to AIP seen at the Malcom Randall VA Medical Center in Gainesville, Florida, were evaluated between March and December of 2018 on an Medtronic Minimed 670G Insulin Pump System. We collected demographic as well as clinical data before and after the initiation of AIP, including standard insulin pump/CGM information (sensor wear, time in target glucose range, time in automated mode, other).
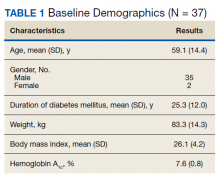
Results: At the time of the initiation of AIP, the mean (SD) age of patients was 59.1 (14.4) years; 35 identified as male and 2 as female. The mean (SD) duration of T1DM was 25.3 (12.0) years. Patients transitioned from either insulin injections or other non-AIP pump to AIP safely—there was no increase in hypoglycemia, and the mean (SD) hemoglobin A 1c decreased from 7.6% (0.8) to 7.3% (0.80) by the second follow-up visit.
Conclusion: In this real-world study, AIP use was both safe and viable as a tool for T1DM management with older veterans. This technology further engaged veterans in monitoring their blood sugars and achieving more optimal glycemic control. Future long-term, larger studies are much needed in this setting.
Statistics
Comparisons of changes in continuous variables between groups were performed by an analysis of covariance (ANCOVA), adjusting for baseline levels. Fisher exact test (χ2) and unpaired t test were used to compare group differences at baseline for categorical and continuous variables, respectively, while Wilcoxon rank sum test was used for nonnormally distributed values. Changes in continuous measures within the same group were tested by paired t test or Wilcoxon matched-pairs signed rank test when applicable. Analyses were performed using Stata 11.0.
Results
Thirty-seven veterans with T1DM using AIPs in 2018 were evaluated at baseline and at follow up visits (Tables 1 and 2). Time frame for follow-up was approximately 3 months, although there was some variation. Of note, the mean weight and BMI corresponded to mostly lean individuals, consistent with the diagnosis of T1DM.
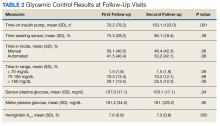
Time below target range hypoglycemia (sensor glucose < 70 mg/dL) remained low at each follow-up visit (both 1.5%). Percentage of time in automated mode increased from first to second follow-up visit after initiation of AIP (41% vs 53%, P = .06). Percentage of sensor wear numerically increased from first to second follow-up visit (75% vs 85%, P = .39), same as time in range, defined as sensor glucose 70 to 180 mg/dL, from first to second follow-up visit (70% vs 73%, P = .09). Time above range, defined as sensor glucose > 180 mg/dL, demonstrated a strong trend toward decreasing between follow-up appointments (29% to 25%; P = .09). HbA1c decreased from 7.6% to 7.3% (P = .005).
About half of the patients (18 of 37) reported hypoglycemia unawareness before the initiation of the 670G AIP. On follow-up visit 61% (11 of 18) reported significant improvement in awareness. Of the remaining 18 patients who reported normal awareness before automated mode, 17% (3 of 18) described a new onset unawareness.
Discussion
This study evaluated the safety of adopting a new DM technology in the real world of an outpatient VA clinic. To the best of our knowledge, this is the first study evaluating the use of AIP specifically in a population of middle-aged veterans with longstanding T1DM. After a mean 7 months of follow-up, participants accepted AIP use as evidenced by increased sensor wear over time and experienced improvements in DM measures that indicate successful use (ie, time in automated mode, which represents reduced glycemic variability). These results show success of an AIP approach in a demographically older group of patients.
AIP has been shown to have positive effects on glycemic control such as time in target glucose range (goal ≥ 70%). In our relatively small pilot study, there was trend for an improvement in the time in range from the first to second clinical follow-up visit, suggesting true patient involvement with the use of the device. Studies involving overall younger cohorts have proved that AIP technology is safe and efficacious for outpatient management of T1DM.7,10,12,13 However, they were all conducted under the safety of a research setting, and trials enrolled a younger population believed to adapt with more ease to this new technology. Tauschmann and colleagues performed a multicenter, parallel randomized controlled trial that compared hybrid closed-loop AIP therapy with sensor-augmented pump therapy in patients with suboptimal T1DM control.12 Results showed that the hybrid closed-loop system increased the time that the glucose concentration was within the target range (70-180 mg/dL) from 54% in the sensor-augmented pump group to 65% on the closed-loop system (P < .001). A small but significant improvement in HBA1c (from 8.0 -7.4%) and low rates of hypoglycemia (2.6% of time below 70 mg/dL) were also noted.12
A similar benefit was observed in a 2019 landmark study by Brown and colleagues of 168 patients with T1DM at 7 university medical centers who were treated for 6 months with either a closed-loop system (closed-loop group) or a sensor-augmented pump (control group) in a parallel-group, unblinded, randomized trial study.13 Mean (SD) time in the target range increased in the closed-loop group from 61% (17) at baseline to 71% (12) during the 6 months. HbA1c decreased from 7.4 to 7.1% and time ≤ 70 mg/dL was just 1.6%. However, only 13% of patients were aged ≥ 40 years in the study by Tauschmann and colleagues, and mean age was 33 years in the Brown and colleagues study.12,13 In contrast, the mean (SD) age in our study was 59 (14) years. Our pilot study also showed comparable, or somewhat better results, as mean time in target range was 72%, HbA1c was 7.3%, and time ≤ 70 mg/dL was just 1.5%.