Evaluation of Pharmacologic Interventions for Weight Management in a Veteran Population
Background: Veterans are disproportionately impacted by weight-related morbidity: 40% of veterans are categorized as obese and an additional 38.5% are overweight. Medications are recommended as an adjunct to lifestyle and dietary changes. Guidelines recommend 7 weight management medications, including orlistat, liraglutide, phentermine, phentermine/topiramate, lorcaserin, and naltrexone/bupropion.
Methods: A single-center, retrospective chart review was conducted for patients who started weight management medications at Veteran Health Indiana in Indianapolis. The primary outcomes included total weight loss and weight loss as a percentage of baseline weight at 3, 6, 12, and > 12 months of therapy. Secondary outcomes included weight loss of 5% from baseline, rate of successful weight maintenance after initial weight loss of 5% from baseline, adverse drug reaction monitoring, and use of weight management medications across clinics at this site.
Results: The absolute weight difference over 12 months of weight management medication therapy was 15.8 kg. At each time point, weight loss was found to be statistically significant when compared with baseline ( P < .001). Average weight change was greatest with orlistat (−25.9 kg) and naltrexone/bupropion was associated with a gain of 2.1 kg over the duration of the study. A majority of the patients analyzed lost the guideline-recommended 5 to 10% from baseline while taking weight management medication.
Conclusions: Weight management medications in a veteran population produced initial weight loss consistent with previous studies. However, there is room for improvement in follow-up strategies to promote greater weight maintenance after initial weight loss. Considering the high health care costs, personal burden, and potential long-term complications associated with obesity, efforts to promote continued development of programs that support weight management and maintenance are imperative.
At the time of study, orlistat, liraglutide, phentermine/topiramate,
Patients were included in the study if they received a prescription of any 1 of the 5 available medications during the enrollment period. Patients were excluded if they received a prescription from or were treated by a civilian health care provider, if they never used the medication, or if their weight loss was attributed to a cancer diagnosis. These criteria produced 86 patients of whom 96 unique weight loss prescriptions were generated. Data were collected for each instance of medication use so that some patients were included multiple times. In this case, data collection for the failed medication ended when failure was documented, and new data points began when new medication was prescribed; all data collected were per medication, not per patient. This method was used to account for medication failure and provide accurate weight loss results based on medication choice within this institution.
The primary outcomes included total weight loss and weight loss as a percentage of baseline weight at 3, 6, 12, and > 12 months of therapy. Secondary outcomes included weight loss of 5% from baseline, rate of successful weight maintenance after initial weight loss of 5% from baseline, adverse drug reaction (ADR) monitoring, and use of weight management medications across clinics at VHI.
Demographic data included race, age, sex, baseline weight, BMI, and comorbid medical conditions. Comorbidities were collected based on the most recent primary care clinical note before initiating medication. Medication data collected included medications used to manage comorbidities. Data related to weight management medication included prescribing clinic, reason for medication discontinuation, or bariatric surgery intervention if applicable.
Efficacy outcome data included weight and BMI across therapy duration. Safety outcomes data included heart rate, BP, and ADRs that resulted in medication discontinuation as documented in the electronic health record (EHR).
We used descriptive statistics, including mean, standard deviation (SD), range, and percentage. For continuous data, Kruskal-Wallis tests were used because of nonparametric data distribution among the different medications with a prespecified α = 0.05. With the observed sample sizes and SDs in this study, post hoc poststudy power calculations showed that the study had 80% power at a 5% significance level to detect weight changes of 8.6 kg, 7.3 kg, and 12.4 kg at 3, 6, and 12 months, respectively, using nonparametric tests.
Results
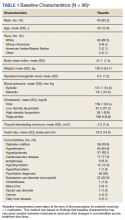
A total of 86 patients were identified based on prescription fills, which produced 99 unique instances of medication use. Of the 99 identified, 3 met exclusion criteria and were not included in the final analysis. Among included veterans, 16 were female and 80 were male (Table 1). Most of those included identified as White race (86%), male (83%), and mean age 53 years. At baseline, mean weight was 130 kg and mean BMI 41.