Implementation and Evaluation of a 90-Minute Rituximab Infusion Protocol at the Richard L. Roudebush VA Medical Center
Background: The use of IV rituximab for the treatment of a variety of malignant and nonmalignant indications has been associated with significant challenges related to time and labor. To help alleviate some of these logistic challenges, institutions have implemented protocols to shorten the time in which rituximab is infused. The purpose of this study was to support the safe implementation of a 90-minute rapid infusion protocol for rituximab at the Richard L. Roudebush VA Medical Center (RLRVAMC).
Methods: A 90-minute rituximab protocol was developed, and proactive measures were taken to educate physicians, pharmacists, and nurses on ordering, processing, compounding, and administering rituximab. A weekly report of patients who received rituximab at RLRVAMC was generated November 1, 2018 through April 1, 2019. Patients then were screened for rapid infusion of the drug based on eligibility criteria, and health care providers (HCPs) were notified. After each patient received a rapid infusion, a retrospective chart review was performed to evaluate patient tolerability and assess for any safety concerns that would require protocol modification. The primary endpoint for this study was the incidence of grade 3 and 4 infusion-related reactions (IRRs) associated with rapid infusions of rituximab based on the Common Terminology Criteria for Adverse Events Version 5.0.
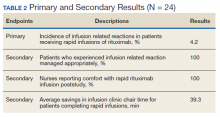
Results: Eleven patients received 24 rapid infusions of ritux imab. Of these infusions, 1 (4.2%) resulted in a grade 3 IRR; no infusions resulted in a reaction of grade ≥ 4. The use of rapid infusion of rituximab when compared with nonrapid infusion saved 39.3 minutes on average per patient.
Conclusions: The proactive measures that were used to im plement the rapid infusion rituximab protocol improved HCP prescribing rates, nursing satisfaction, and the management of IRRs. This study confirmed appropriateness of rapid administration of rituximab in this veteran population and has increased interest in implementing other rapid infusion protocols.
Results
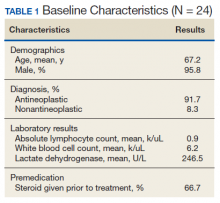
Between November 1, 2018 and April 1, 2019, 11 patients received a total of 24 rapid infusions of rituximab. The majority of patients included in the study were older males, and the most common indication for rapid infusion was follicular lymphoma (Table 1).
Primary Endpoint
All patients who received a rapid infusion of rituximab were reviewed in the analysis of the primary and secondary endpoints. Among the 24 rapid infusions of rituximab, 1 infusion was stopped due to the patient experiencing a grade 3 IRR according to criteria from CTCAE Version 5.0. The patient was found to have dysphagia at baseline and experienced severe symptoms in the days following the first infusion that put the patient at high risk for subsequent infusion related concerns. Eligibility criteria for the 90-minute protocol were updated based on these findings. No patient experienced a grade 4 or 5 IRR. The remaining 23 infusions were well tolerated by the patients with no clinically significant events.
Secondary Endpoints
The patient who experienced a grade 3 IRR to rituximab received proper treatment by infusion clinic nurses according to the RLRVAMC hypersensitivity protocol. Patients who received rapid infusions of rituximab had a mean length of infusion of 95.0 minutes. This was in contrast to the mean time of each patient’s previous nonrapid infusion of 134.3 minutes. The difference between the 2 values equated to a savings in infusion clinic chair mean time of 39.3 minutes per patient.
Nurses were asked whether they had prior experience administering rituximab via 90-minute infusion and whether they felt comfortable administering a 90-minute rituximab infusion. Before the live education session, none of the nurses surveyed had prior experience or felt comfortable administering rituximab over 90 minutes. When the nurses were surveyed poststudy, all reported that they were experienced administering rituximab and felt comfortable with the process (Table 2).
Discussion
The infusion of rituximab has been associated with significant challenges related to the time and labor required. Although a vast number of institutions across the country now infuse the medication over an abbreviated time, HCP concerns for patient safety and appropriate use of hypersensitivity protocol in a veteran population delayed implementation at RLRVAMC. The results from this quality improvement initiative highlight the positive impact of the proactive measures that were used to implement the rapid infusion protocol for rituximab on improving HCP prescribing rates, nursing satisfaction, and appropriate management of IRRs.
Rapid infusion saved on average 39.3 minutes per patient in infusion clinic chair time. Each successful rapid infusion of rituximab potentially opened additional time in clinic for ≥ 1 patients to receive an infusion therapy. The RLRVAMC usually operated at maximum capacity, so the ability to accommodate more patients helped decrease hospital admittances for time-sensitive infusions.
The initial criteria used to screen patients to determine whether a rapid infusion of rituximab would be appropriate was based on inclusion and exclusion criteria for past studies on the same subject.3-5 The incidence of hypersensitivity reactions associated with study participants who received rapid rituximab infusions also resembles past research done on the subject, which is important to note due to prior misconceptions of staff at the institution of a higher risk of reaction in this specific veteran population. One patient with RA experienced a grade 3 IRR in this study. Although this patient met the original inclusion criteria, the patient had baseline dysphagia, and following the first infusion, reported to the emergency department (ED) with symptoms of delayed anaphylaxis. In this case, the order for rapid infusion was placed in advance and the prescriber was unaware of the ED visit. Based on this event, eligibility criteria for 90-minute rituximab infusions were updated to include additional information specifying that candidates for a rapid infusion also may have no baseline airway compromise. This hypersensitivity reaction also highlighted the need for decision support technology to assist HCPs in patient selection as well as empowering nursing and pharmacy staff to identify concerns once they place orders.
Over the course of the study, investigators assisted the HCPs with preparation of orders for the rapid infusion of rituximab for antineoplastic indications. Due to feasibility issues with this practice moving forward, order sets containing rituximab were updated to include a 90-minute option. This created a more standardized process that allowed HCPs to screen potential patients on their own. The expectation is that HCPs will be more likely to order 90-minute infusions for eligible patients in the future with this efficient and safer process.