Screening Tool to Reduce Anticoagulant Clinic Encounters
Objective: Patients prescribed warfarin for the prevention of stroke in atrial fibrillation at the Fayetteville Veterans Affairs Heath Care Center are managed by a clinical pharmacy specialist (CPS) in the Anticoagulation Clinic. Patients prescribed a direct oral anticoagulant (DOAC) for the same indication are followed by a CPS within the patient aligned care team. A screening tool was developed to identify candidates who could switch from warfarin to DOAC therapy. The purpose of this quality improvement project was to determine the impact of a screening tool on the average number of monthly Anticoagulation Clinic encounters.
Methods: The impact of the screening tool to effect the num ber of clinic encounters was studied for 3 months prior to and 2 months following an 8-week screening period. The screening tool, created to determine the eligibility of patients to switch to DOAC, was developed based on US Department of Veterans Affairs Pharmacy Benefits Management Service guidance. Eligible patients were counseled on DOACs and given the opportunity to shift therapies. The total number of encounters associated with all anticoagulant patients, including those who changed to DOACs, was recorded.
Results: During the 3 months prior to screening, an average of 476 encounters per month were documented. For 2 months following screening, an average of 546 encounters per month were recorded. Seventy additional monthly encounters were observed after the screening tool implementation ( P = .15). Thirty patients chose to switch to DOAC therapy; there were 75 fewer encounters among these 30 patients in the postscreening period, a reduction of 70.1% ( P = .01).
Conclusions: The DOAC screening tool was unsuccessful in reducing the overall number of Anticoagulation Clinic encounters. However, it was determined that when patients switched from warfarin to a DOAC, encounters were reduced. Several confounding factors influenced study results.
Methods
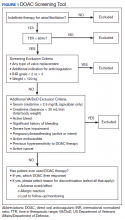
As this undertaking was considered to be a quality improvement project, institutional review board approval was not required. During an 8-week screening period (August to September 2018), the DOAC screening tool was implemented into the Anticoagulation Clinic workflow. This screening tool (Figure 1) was established based on VA Pharmacy Benefit Management (PBM) Service’s Criteria for Use for Stroke Prevention in Nonvalvular Atrial Fibrillation, a national set of standards used to determine appropriate candidates for DOAC therapy.3
Exclusion criteria included patients with INR goals < 2 or > 3, patients with a diagnosis of VTE, and patients with weight > 120 kg. Patients with a diagnosis of VTE were excluded due to the variability in therapy duration. Weight cutoffs were based on recommendations by the International Society on Thrombosis and Haemostasis. Due to a lack of available data, it was suggested that clinical judgment be used in patients whose weight was > 120 kg.4
During the screening period, weekly TTR reports identified patients in the clinic who had TTR < 60%. When a patient with a TTR report results of < 60% also had a scheduled appointment within a week, a CPS then further reviewed patient eligibility using the DOAC screening tool. On arrival for an appointment, the eligible patient was counseled on DOAC medications and the differences between warfarin and DOACs, including monitoring requirements. Patients had the option to switch to DOAC therapy or remain on warfarin.
The change in the average number of monthly Anticoagulation Clinic encounters for 3 months prior to the screening period (May to July 2018) and 2 months following screening (October to November 2018) was evaluated to measure the impact of the DOAC screening tool. The total number of encounters in the clinic was assessed using the monthly VA reports and were averaged for each period. Then data from the 2 periods were compared.
The monthly encounter reports, a data tool that monitors the number of unique visits per veteran each calendar month, also were used to generate a secondary endpoint showing the number of encounters in the Anticoagulation Clinic associated with patients who switched to a DOAC, including visits prior to changing therapy, and before and after the screening period.
Student’s t test was used to compare the change in encounter frequency before and after screening tool implementation for both primary and secondary endpoints. α was defined as .05 a priori. Continuous data were presented as means and standard deviations. Data were calculated with Microsoft Excel 2016.
Results
For the 3 months before the 8-week screening period, an average of 476 Anticoagulation Clinic encounters per month were documented. Two months of data following the screening period averaged 546 encounters per month. There were an average of 70 additional encounters per month after screening tool implementation (P = .15), reflecting the study’s primary objective.
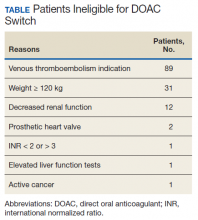
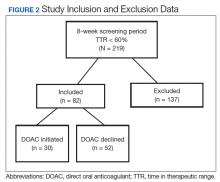
A total of 219 patients in the Anticoagulation Clinic were identified as having a TTR report results of < 60% during the 8-week screening period (Figure 2). Eighty-two of those patients (37.4%) were considered eligible to switch from warfarin to DOAC therapy. Thirty of those eligible patients (13.7%) switched to a DOAC. A total of 107 clinic encounters (22.5%) was associated with these 30 patients prior to screening and 32 associated encounters (5.9%) following screening (P = .01). Of the remaining 137 patients (62.6%) who were ineligible for DOAC therapy, the most common reason for disqualification was a diagnosis of VTE (Table).