The Effects of Ranolazine on Hemoglobin A1c in a Veteran Population
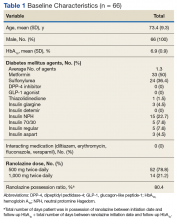
Subjects were eligible for inclusion in this study if they were aged ≥ 18 years, had a diagnosis of type 2 DM, and received their first prescription of ranolazine at a VAMC from January 1, 2008 through March 31, 2015. Exclusion criteria included subjects with no baseline HbA1c (defined as the HbA1c result closest to the ranolazine initiation date and within 90 days before to 14 days after ranolazine initiation), no follow-up HbA1c (defined as the first HbA1c result within 60 to 180 days after the ranolazine initiation date), any change to their DM medication regimen during the follow-up period, or who discontinued ranolazine prior to collection of the follow-up HbA1c.
Data were collected from the electronic health record (EHR) for each subject from 6 months prior to the ranolazine initiation date through 6 months after the ranolazine initiation date. The ranolazine initiation date was defined as the date ranolazine was picked up in person at a VAMC pharmacy or 7 days after the date filled for medications sent by mail.
The primary endpoint of this study was the change in HbA1c after ranolazine initiation. The secondary endpoint was the percentage of study subjects achieving HbA1c < 7% and < 8% before and after the initiation of ranolazine.
To achieve 80% power to detect a change in HbA1c of 0.4%, a sample size of 52 patients was required. For the primary endpoint, a paired t test was used to test for statistical significance. The McNemar test was used to evaluate for a significant change in subjects achieving an HbA1c < 7% and HbA1c < 8% with the initiation of ranolazine.
Results
A total of 523 patients were evaluated for study inclusion, of which 66 patients were included (Figure). The most common reasons for exclusion included no HbA1c at baseline and changes to the DM medication regimen during follow-up.
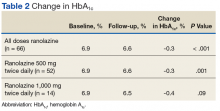
Ranolazine at any dose was associated with a change in HbA1c of -0.3% (P < .001).
A dose of 500 mg ranolazine twice daily also was associated with a significant decrease in HbA1c by 0.3% (P = .001). A significant increase in veterans achieving HbA1c < 7% after ranolazine initiation was observed (42.3% before ranolazine initiation vs 73.1% after ranolazine initiation; P = .001), and a nonsignificant increase in veterans achieving HbA1c < 8% was observed (82.7% before ranolazine initiation vs 90.4% after ranolazine initiation, P = .37).
At a dose of 1,000 mg twice daily, a 0.4% decrease in HbA1c was observed. However, this result was not found to be statistically significant (P = .09), and the study was underpowered to detect a significant change in HbA1c at this dose.
Hypoglycemia was not reported in a majority of study patient progress notes; thus, it was not evaluated further.
Discussion
In this study of a veteran population, ranolazine was associated with an HbA1c decrease of 0.3%. This change is less than that observed in previous studies, which may be related to a lower baseline HbA1c for the patients in this study. In addition, a greater percentage of veterans achieved an HbA1c < 7% after initiation of ranolazine compared with that of the baseline.
To the authors’ knowledge, this is the first study evaluating ranolazine and HbA1c in a veteran population. It also is the first study to demonstrate an association between HbA1c lowering and ranolazine at a dose of 500 mg twice daily. These results suggest that in patients with chronic angina and type 2 DM, ranolazine could potentially play a dual role in therapy.