Gabapentin Use in Acute Alcohol Withdrawal Management
Secondary Endpoints
On average, the total number of days spent on CIWA-Ar protocol was 3.8 (median 4, ± 1.5) in group 1 compared with 4.1 (median 4, ± 1.6) in group 2. Rate of CIWA-Ar protocol discontinuation for patients in group 1 and group 2 is shown in Figure 3.
Discussion
The purpose of this project was to evaluate gabapentin use at VAPORHCS for alcohol withdrawal and evaluate the impact on symptom management. Patients who were started on gabapentin on the initiation of the alcohol withdrawal protocol received less lorazepam dosing compared with patients who received only lorazepam for symptom management for alcohol withdrawal. Except for day 3, average lorazepam dosage per day on the alcohol withdrawal protocol was lower in patients who were also taking gabapentin.
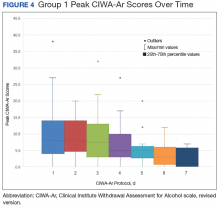
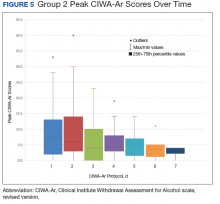
This trend also can be seen in the recorded peak CIWA-Ar scores per day as illustrated in Figures 4 and 5.
Limitations
Prior to evaluation, power analysis was not calculated to estimate an appropriate sample size necessary to determine statistical significance. Results from this evaluation are not definitive and are meant to be hypothesis generating for future analysis.
There were several limitations that were identified throughout this project. For this review, history and extent of patient’s prior alcohol use was not assessed. Therefore, the degree of symptom severity in which patients may have experienced during withdrawal may not have been adequately matched between groups. The inherent subjectivity of CIWA-Ar scoring was considered a limitation because scores were determined by clinical interpretation among various nursing staff. As this was a retrospective review, exact timing of medications administered as well as additional supportive care measures, such as ancillary medications for symptom management, were not accounted for and controlled between groups.
Patients presenting to the emergency department or from a facility outside of VAPORHCS for acute AWS may have had incomplete documentation of the onset of symptoms on presentation or of the medications administered prior to being admitted, which may have confounded initial CIWA-Ar scoring and total duration required to be on a withdrawal protocol. Some patients may have received benzodiazepines at initial presentation prior to gabapentin initiation and may have inaccurately reflected its efficacy potential to manage symptoms without the need for lorazepam.
There were 10 patients that were identified who received gabapentin on the alcohol withdrawal protocol and did not receive any lorazepam. This retrospective review could not be determined whether these patients did not require lorazepam because initiating gabapentin reduced severity or simply because their withdrawal symptoms were not severe enough to warrant the need for lorazepam, regardless of gabapentin use.
Gabapentin dosing was not standardized among patients, averaging from 100 mg to 3,600 mg per day. This wide variation in dose may have influenced the requirement of lorazepam needed for symptom management in patients receiving minimal doses or AEs experienced in patients who received large doses. Initiation and/or select dosing of gabapentin may have been dependent on the experience of the provider and familiarity with its use in alcohol withdrawal management. Interestingly, patients with a history of withdrawal seizures (13%) were identified only within the lorazepam-only group. This could suggest that patients with prior symptoms of severe alcohol withdrawal were selected to receive lorazepam-only at the discretion of the provider.
Existing literature investigating gabapentin utilization in alcohol withdrawal has demonstrated benefit for patients with mild-to-moderate symptoms in both inpatient and outpatient studies. However, supporting evidence is limited by the differences in design, methods, and comparators within each trial. Leung and colleagues identified 5 studies that utilized gabapentin as monotherapy or in combination with other agents in alcohol withdrawal.13 Three of these studies were performed within an inpatient setting, each differing in trial design, inclusion/exclusion criteria, intervention, and outcomes. Gabapentin dosing strategies were highly variable among studies. Collectively, the differences noted make it difficult to generalize that similar outcomes would result in other patient populations. The purpose of this project was to evaluate gabapentin use at VAPORHCS for alcohol withdrawal and evaluate the impact on symptom management. Future projects could be designed to draw more specific conclusions.