Decentralized vs Centralized Pharmacist Treatment of Patients With Atrial Fibrillation Managed With Direct Oral Anticoagulants
Methods
This single-center, retrospective anticoagulant-use evaluation covered 2 study periods between November 1, 2011 and October 31, 2013. Study approval was obtained from the institutional review board of the Medical University of South Carolina and the research and development committee of RHJVAMC. The study population consisted of veterans who had a diagnosis of AF and received at least 3 outpatient prescription fills of a 30-day supply of dabigatran at RHJVAMC during either or both of the study periods. Patients were excluded if they were pregnant or planning to become pregnant or were incarcerated at any time during the study period. Dabigatran was selected because it was the first DOAC added to the local VA formulary before the start of this study.
Patients who met the inclusion criteria were separated into 2 groups based on the dates of their prescription fills. The precentralization group included patients treated by primary care pharmacists from November 1, 2011 to October 31, 2012; the postcentralization group included patients treated by anticoagulation clinical pharmacy specialists from November 1, 2012 to October 31, 2013. In each group, patients were followed for 1 year during their respective study period. For analysis, patients were included in both study periods if they received at least 3 fills of dabigatran during each period.
Medication possession ratio (MPR), which was used to measure the primary endpoint of adherence, is defined as the proportion of days a patient had dabigatran. The MPR denominator is the total number of days between the first and last prescription refill dates within the 52-week study period; the numerator is calculated by summing the days' supply for all but the last filling of the medication during each respective period. Nonadherence was defined as an MPR < 0.8 (or 80%), which has been used to define poor adherence in the literature.12 The authors calculated all patients' mean MPRs and compared them to determine statistical significance by repeated-measures linear regression. Descriptive statistics on proportion of patients in each study group with MPR < 0.8 were examined. Last, the authors performed a comparative subanalysis of median MPRs to determine whether there was an adherence difference between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC.
The secondary focus of this study was safety outcomes, including any bleeding event or thromboembolism within either study period. A bleeding event was defined as any major or minor bleeding event recognized through ICD-9 codes or any bleeding recorded in the patient's chart and noted during chart review, as well as any serum hemoglobin (Hgb) level decrease of ≥ to 2 g/dL during the study period. Thromboembolism was defined as a thromboembolism recognized through ICD-9 codes or any thromboembolism noted during chart review. Descriptive statistics were reported for this outcome, and a chi-square test was used to compare bleeding events between groups to determine significance.
The tertiary focus of this study was clinical efficiency as determined by number of primary care pharmacist visits during each study period. Primary care pharmacist visits were included for all primary care pharmacists in primary care clinics at the main hospital and in all 6 CBOCs.
For statistical analysis α was set at 0.05, and P < .05 was considered statistically significant. SAS Enterprise Guide software (Cary, North Carolina) was used for all statistical analyses.
Results
An initial data pull was completed from the RHJVAMC prescription records database for patients who had ≥ 3 prescriptions of dabigatran filled for treatment of AF during the study period, which yielded 65 unique patients. There were 34 patients in the precentralization group and 55 patients in the postcentralization group. Twenty-four unique patients were included in both study groups.
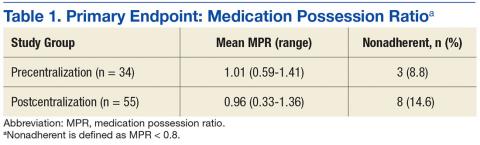
Mean MPR was 1.01 (range, 0.59-1.41) for the precentralization study period and 0.96 (range, 0.33-1.36) for the postcentralization period (Table 1). The difference was not statistically significant (P = .91). Number of patients considered nonadherent (MPR < 0.8) was 3 (8.82%) in the precentralization group and 8 (14.6%) in the postcentralization group.
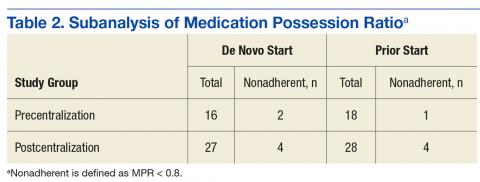
The primary endpoint subanalysis compared the median MPRs for the patients initially started on dabigatran at RHJVAMC (de novo starts) and the patients who were started on dabigatran before receiving it at RHJVAMC (prior starts). In each group, number and percentage of patients determined to be nonadherent by MPR were evaluated as well. De novo patients received initial assessment, counseling, and a dabigatran prescription from RHJVAMC pharmacists before or during the study period, and prior patients were initially prescribed dabigatran at another VA facility or at a non-VA facility (Table 2).