Lessons Learned From the RACAT Trial: A Comparison of Rheumatoid Arthritis Therapies
Trial Results
The trial was a 48-week, double-blind, noninferiority trial in which 353 patients with RA who had active disease despite MTX therapy were randomized to a triple therapy regimen of DMARDs (baseline MTX, plus SSZ and hydroxychloroquine) or baseline MTX plus etanercept (Figure 1). Patients who did not have a clinically significant improvement at 24 weeks according to a prespecified threshold were switched in a blinded fashion to the other therapy.
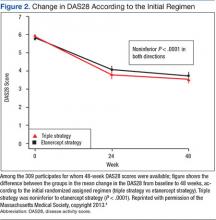
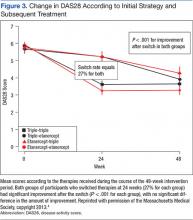
The primary endpoint, the change between baseline and 48 weeks in the DAS28, was similar; thus the strategy of first starting triple therapy was not inferior to first starting etanercept (the change in DAS28 was -2.12 and -2.29 respectively, P < .0001, supporting noninferiority, Figure 2). Both groups had significant improvement over the course of the first 24 weeks (P = .001). A total of 27% of participants in each group switched at 24 weeks (Figure 3). Patients in both groups who switched therapies had improvement after switching (P < .001), and the response after switching did not differ significantly between the 2 groups. Importantly, there were no significant between-group differences in radiographic progression (P = .43), pain and health-related quality of life (QOL), or in medication-associated major adverse events (AEs), although there were numerically more serious infections with etanercept-MTX therapy (12 vs 4). Gastrointestinal AEs were numerically more frequent with triple therapy; whereas infections and skin and subcutaneous AEs were more frequent with etanercept-MTX therapy.
The cost-effectiveness of adding SSZ and hydroxychloroquine to MTX vs adding etanercept to MTX, using a predetermined measurement of QOL, was assessed in this trial.9 These data were initially presented in abstract form at the 2014 American College of Rheumatology national meeting. Considered was the ratio of all the incremental costs between the 2 treatment strategies to the benefits, as measured in quality-adjusted life-years (QALYs), where QOL is measured as an index with 1 being equivalent to full health and 0 being equivalent to death. This incremental cost-effectiveness ratio produces a monetary cost for each QALY, which is an indication of cost-effectiveness and value. Most health care systems currently consider anything that costs < $50,000/QALY to be cost-effective. To be conservative, the trial researchers considered anything up to $100,000 for an additional QALY acceptable.
In the 48-week trial analysis, the use of etanercept first, instead of triple therapy, would incur about $1 million of cost per QALY, far more than the $50,000 to $100,000 deemed to be reasonable value. Biologic therapy use first, had a near-zero chance of being cost-effective and would be cost-effective only after failure of triple therapy; results that were robust to all plausible scenarios.
Economic Implications
As noted in the trial design, economic data were prospectively collected for later analysis. The availability and cost of biologic treatments have become a critical issue. In 2005, it was reported that the U.S. biologics market reached $52 billion and was noted to have an annual growth of 17%.10 In 2011, 8 of the top 20 drugs sold in the U.S. were biologics, and year-to-year biologics spending has grown by 6.6%. In 2013, the top 100 biologics in the U.S. had combined sales of $66.3 billion.11 The researchers analysis demonstrated that using a strategy of triple therapy first could result in health care cost savings in the tens of billions of dollars. Importantly, these savings would occur at the same time as patients were receiving optimal care.
Summary
The major conclusions from the RACAT trial in RA patients with active disease despite MTX are the following: 1. The strategy of first starting the conventional DMARD triple therapy combination is noninferior to first starting etanercept, based on both clinical and radiographic outcomes. 2. The triple therapy group had more minor gastrointestinal events, whereas the etanercept group had more infections. Patients in either group who did not respond well to the initial treatment and switched (27% in both groups) improved significantly after the switch. 3. The economic implications of these findings are significant. The incremental cost differences approached $1 million per QALY.
The VA CSP should be congratulated for supporting and funding this trial, which will inform therapeutic decisions in RA for years to come. These results allow clinicians to provide not only optimal health care for their RA patients, but also maximize the value of their health care.