Implications of Vancomycin Troughs Drawn Earlier Than Current Guidelines
Results
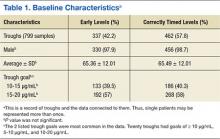
A total of 474 patient charts were reviewed, and 278 met inclusion criteria (196 were excluded). Of the included patients, 799 trough levels were analyzed. Of these, 377 (42.2%) were drawn early. There was no significant difference in the baseline characteristics of the early group vs the correctly timed group (Table 1). Of the early troughs, 190 (56.3%) were drawn prior to the third dose of vancomycin. It was observed that a large portion of these troughs occurred after a vancomycin dose adjustment.
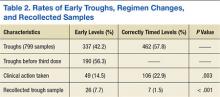
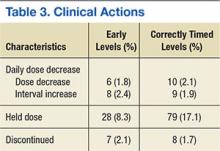
Clinical actions taken after sampling occurred at a rate of 14.5% in the early group and 22.9% in the correctly timed group (P = .003; Table 2). Early troughs led to a 7.7% rate of trough recollection, which was significantly greater than the 1.5% rate in the correctly timed group (P < .001). An analysis of each factor resulting in a clinical action illustrated that the rates of daily dose decrease and discontinued dose were similar between the groups (Table 3). However, the rate of held doses was 8.3% in the early group and 17.1% in the correctly timed group.
This research process yielded some observations. Occasionally a trough was drawn after vancomycin therapy was discontinued and when there was no concern for nephrotoxicity. After the guidelines were published, providers continued to document in CPRS notes to check troughs before the third dose. This incidence decreased over time. Troughs were taken often in patients who were receiving a short course of therapy or who were hemodynamically stable. Finally, documentation of vancomycin regimen changes occasionally did not match the record in the BCMA (in these situations, the BCMA record was used for this study).
Related: Assessment of High Staphylococcus aureus MIC and Poor Patient Outcomes
Discussion
A large portion of trough levels at the JALFHCC were drawn early and did not adhere to the 2009 consensus guidelines. The rates of early troughs in this study and in the Boston, Massachusetts, study are similar.5 However, the 2 studies differed in 1 significant aspect: Clinical actions were taken less often in the early group at JALFHCC, whereas they were taken more often in the early group in the Boston, Massachusetts, study. This dissimilarity could be attributed to a difference in software between the hospitals. In the previous study, trough levels and the time that they were drawn were not displayed together. Thus, clinicians may have been less likely to gauge whether a trough was early. Since this information is available at the JALFHCC, clinicians may have been aware that the trough was early and avoided adjusting treatment (such as holding a dose, as illustrated in the data) based on a falsely elevated trough. This point is further supported by significantly greater amounts of recollected troughs in the early group, suggesting an understanding that the trough was early.
The low trough recollection rate of 7.7% of all early samples could be due to several factors that would prevent a trough redraw. First, medication discontinuation resulting from course completion or sensitivity results would not require further trough monitoring. Second, practitioners may assess the early sample as insignificantly different from a correctly timed one and elect not to redraw the trough. Sometimes a trough was drawn at the correct time, but the time was recorded incorrectly. In this situation, a new trough level would not be necessary. Finally, a lack of sufficient staffing during nights and weekends may result in a delay in interpreting results leading to a missed opportunity for recollection. Additionally, some troughs may not have been redrawn based on a practitioner’s opinion that a trough was not significantly early and did not represent skewed results. Sometimes an incorrect recording of trough draw time reflected that it was taken after vancomycin dosing when it was not.
Specific observations regarding the timing of the trough indicate other possible concerns and areas for improvement. First, providers must cancel future trough orders concurrently with canceling treatment. Second, at the time of publication of the consensus, some providers were slow adopters of the new guidelines. Finally, the IDSA guidelines state that frequent monitoring for short course, lower intensity therapy, or in patients who are hemodynamically stable is not recommended.3 However, troughs were sometimes measured 2 to 3 times weekly in these patients.
Related: Results mixed in hospital efforts to tackle antimicrobial resistance
The data and observations lead to the conclusion that although providers may be able to discern between early and correctly timed troughs, they were not consistently adherent to the 2009 IDSA guidelines. It has been shown that pharmacy involvement of Medicare patients with infections in the intensive care unit has led to better clinical and monetary outcomes.8 Therefore, continued efforts by clinical pharmacists to monitor trough timing can be used to improve adherence and decrease costs (each trough is estimated to cost $16.97).