Efficacy, Safety, and Tolerability of Halobetasol Propionate 0.01%–Tazarotene 0.045% Lotion for Moderate to Severe Plaque Psoriasis in the Hispanic Population: Post Hoc Analysis
Topical corticosteroids are the mainstay of psoriasis treatment; however, safety concerns often limit their use. Combination therapy with tazarotene (TAZ) may optimize efficacy, minimizing safety and tolerability concerns. Although psoriasis affects a diverse patient population, data on the efficacy and safety of topical therapies for the treatment of psoriasis in Hispanic patients are sparse. This post hoc analysis investigated the efficacy, safety, and tolerability of once-daily application, fixed-combination halobetasol propionate (HP) 0.01%–TAZ 0.045% lotion in Hispanic participants with moderate to severe plaque psoriasis. Rapid and sustained reductions in disease severity were found in the Hispanic population treated with HP/TAZ lotion with good tolerability and safety over 8 weeks.
Practice Points
- Although psoriasis is a common inflammatory disease, data in the Hispanic population are sparse and disease may be more severe.
- A recent clinical investigation with halobetasol propionate 0.01%–tazarotene 0.045% lotion included a number of Hispanic patients, affording an ideal opportunity to provide important data on this population.
- This fixed-combination therapy was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
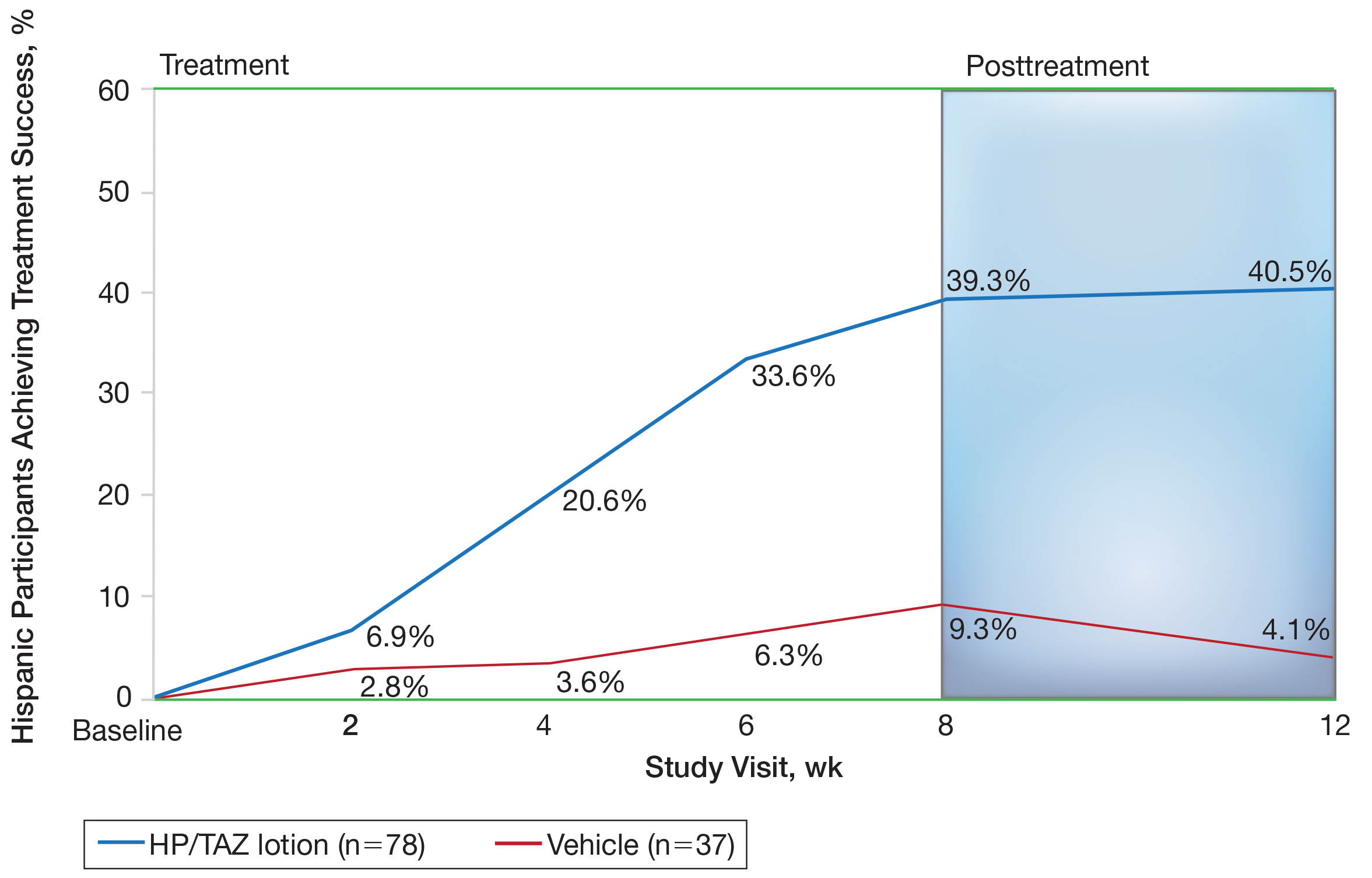
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
,
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.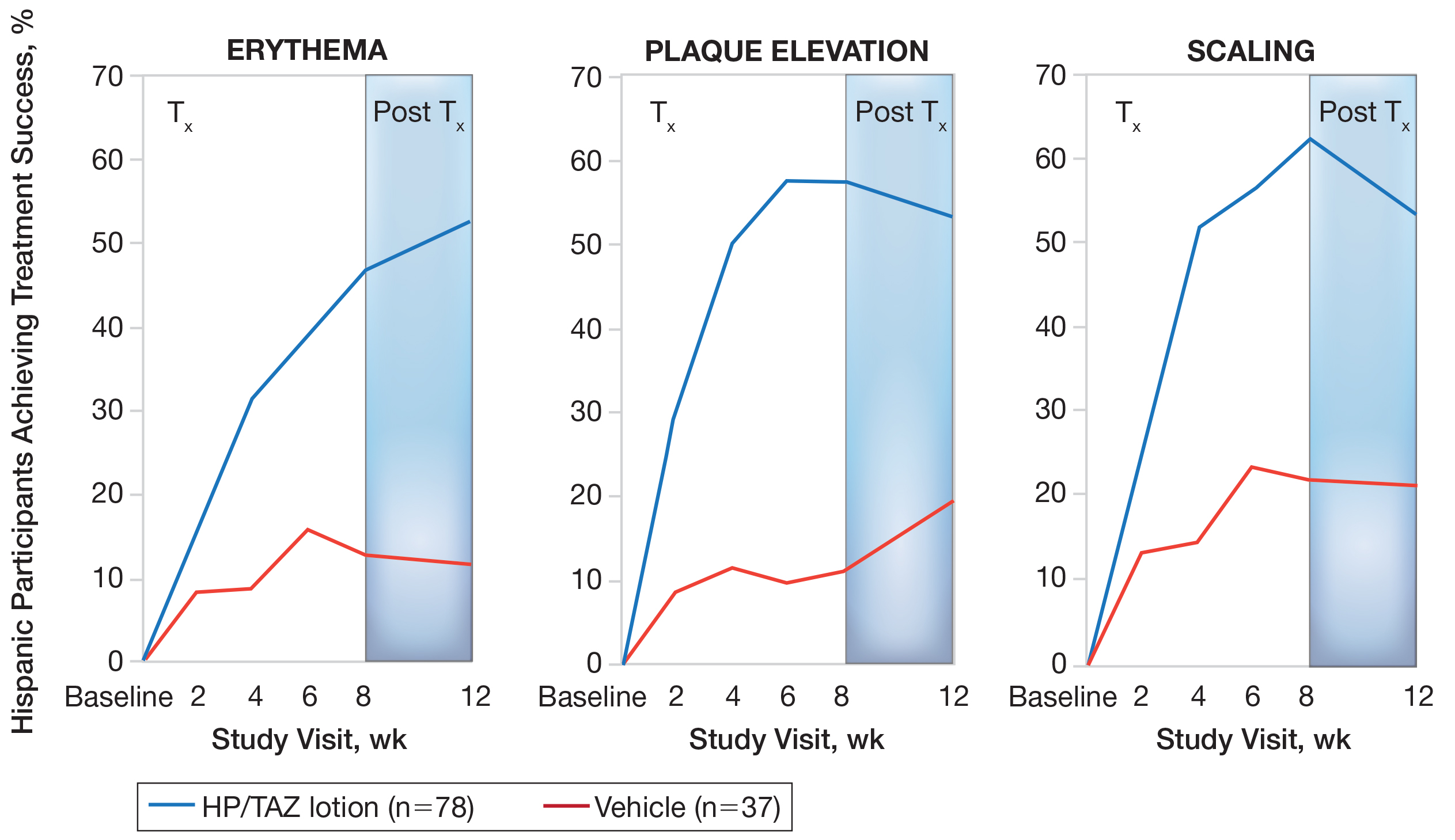
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).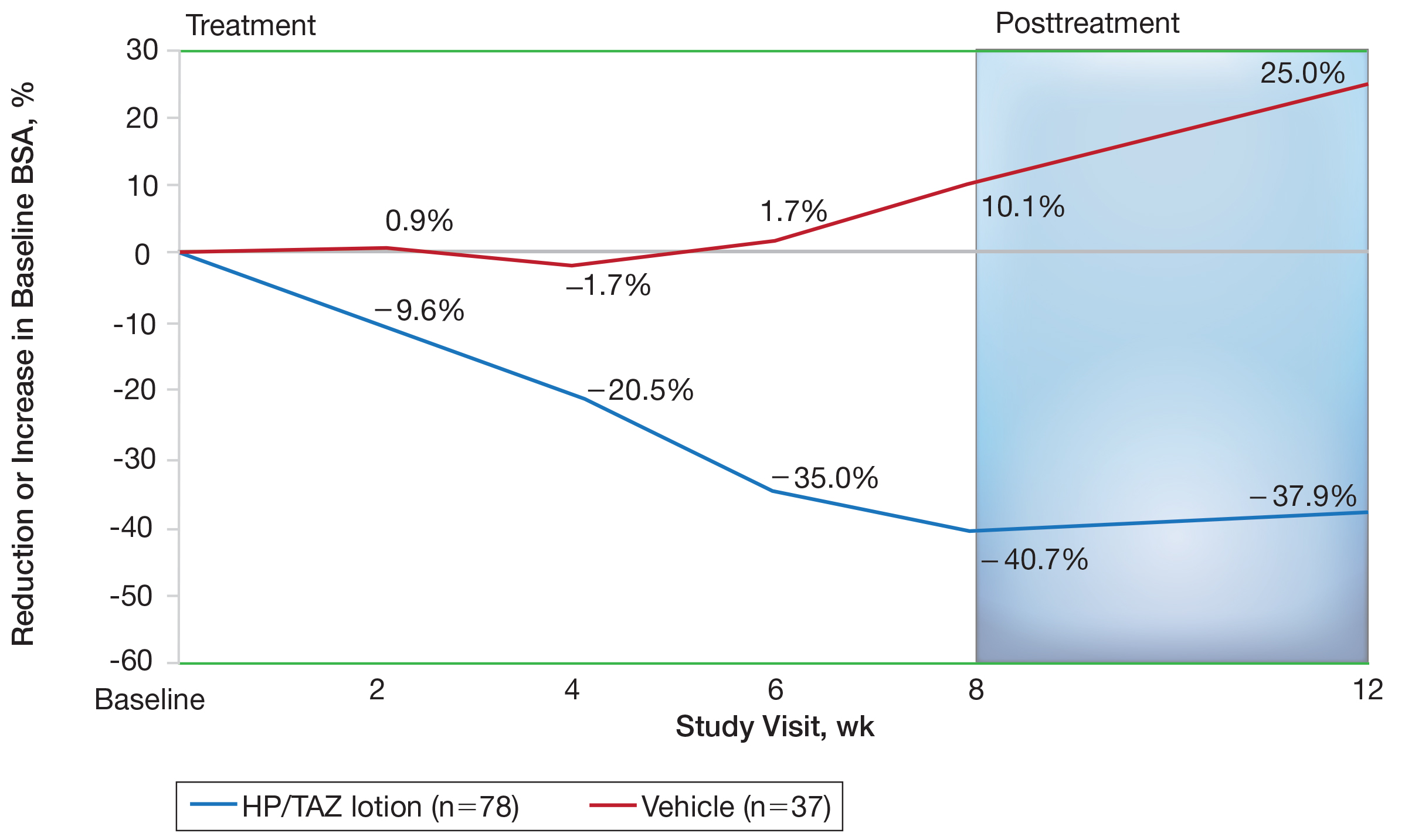
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10 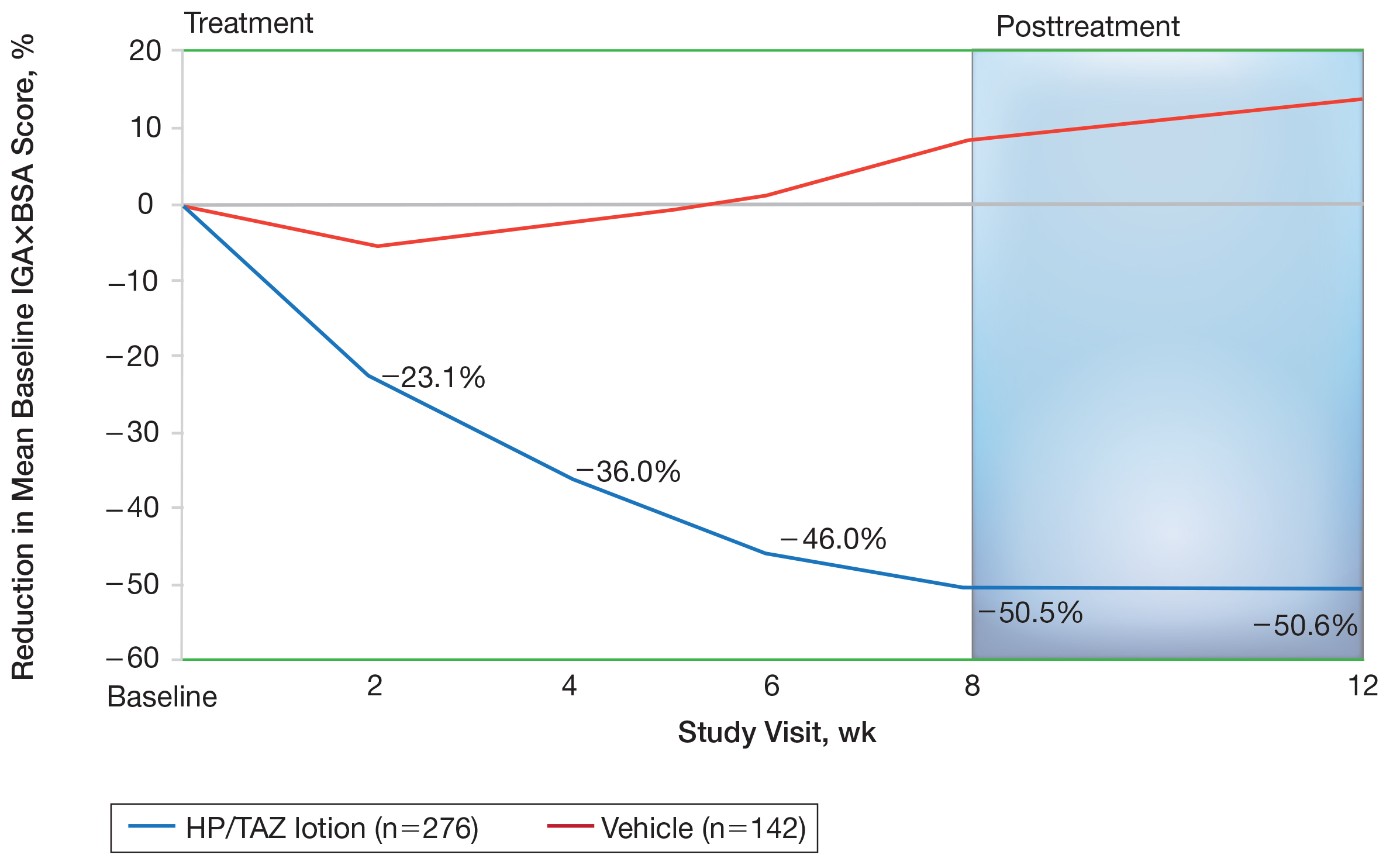
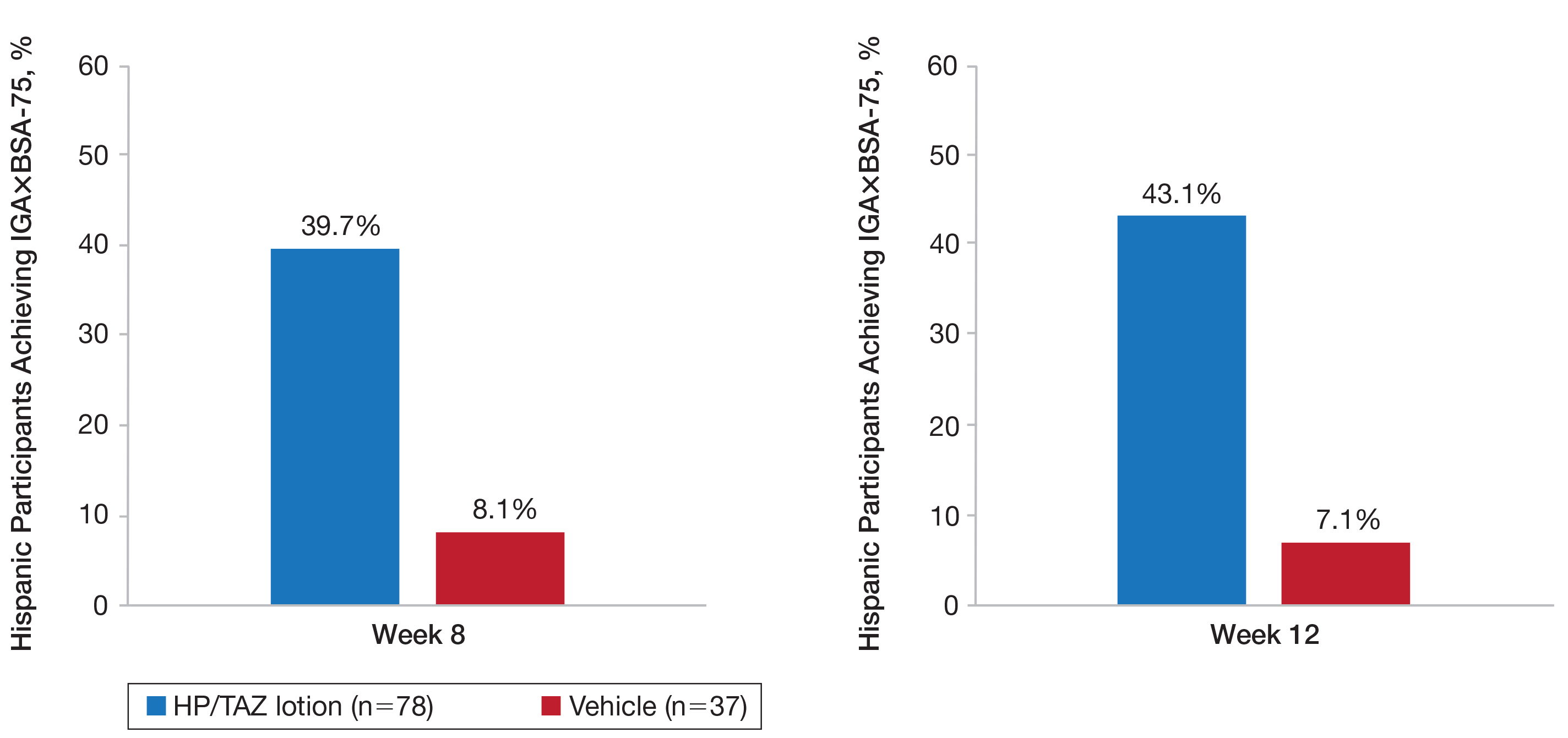
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).