COVID-19 Cycle Threshold/Cycle Number Testing at a Community Living Center
Background: COVID-19 imposes a special risk to the nursing home population, including community living centers (CLCs) for veterans. Cycle threshold/cycle number (CT/CN) values obtained by serial reverse transcriptase polymerase chain reaction (RT-PCR) testing could yield valuable information about viral load and potential infectiousness. Serial testing for COVID- 19 with CT/CN correlates in a nursing home population during an outbreak has not yet been reported in the literature.
Methods: A retrospective review of serial RT-PCR testing for COVID-19 during an outbreak at a CLC was performed from March 28 to April 4, 2020, with follow-up of identified patients until November 10, 2020. Testing was performed on the Abbot m2000 or Cepheid platform.
Results: Of 80 patients tested, 25 (31%) were positive for COVID-19. CT/CN values corresponded to the infection course as expected.
Conclusions: Repeat testing for COVID-19 accompanied by CT/ CN values could provide clinical and epidemiologic information about the likely stage of the patients’ disease course, which may aid public health measures and clinical management.
COVID-19, caused by SARS-CoV-2, is more severe in individuals with underlying illnesses. Because complete social distancing might be more difficult in nursing homes and community living centers (CLCs), public health leaders and clinicians have been concerned about the epidemiology and disease course in nursing homes even before the COVID-19 pandemic.1-7 A report of a COVID-19 outbreak in a nursing home facility in King County, Washington, documented a 33.7% overall fatality rate for residents and 52.4% among the most critically ill.4,5 The experience at King County, Washington, shows that proactive steps to identify, monitor, and apply preventive control measures is important for future outbreaks.5
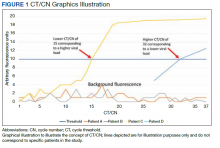
Reverse transcriptase polymerase chain reaction (RT-PCR) testing produces a cycle threshold (CT) or cycle number (CN) that correlates with viral load and infectiousness. 8-14 CT/CN represents the number of RT-PCR cycles required for the fluorescent signal to cross the detection threshold (exceed background level) and is inversely proportional to the viral load. Effectively, the higher the viral load, the lower the CT/ CN value (Figure 1). Tracking CT/CN values was not documented in the Washington nursing home outbreak. Reports of COVID- 19 testing in CLCs during outbreaks are sparse, and CT/CN values and demographic distribution of these veterans has not been reported.15 The CLC veteran population, with known higher vulnerability to infection and chronic diseases, is epidemiologically different from the general nursing home population.15-18 To address these literature gaps, we present the first report of COVID- 19 testing with CT/CN value correlations in the high-risk veteran CLC population.
Methods
A retrospective review of all COVID-19 CT/CN testing at the Corporal Michael J. Crescenz Veterans Affairs Medical Center (VAMC) CLC in Philadelphia, Pennsylvania, from March 28, 2020, to April 24, 2020, was performed with a US Department of Veterans Affairs (VA) Veterans Health Information System Architecture VistA/FileMan search. Only veteran residents were included in this review. Data collected included initial and serial test results, CT/CN on positive test results, test dates, testing platform used, demographic information (age, self-reported ethnicity, and sex), and clinical follow-up information. Health records were reviewed retrospectively to identify death, the first day after diagnosis with no documented symptoms, or hospitalization status.
RT-PCR testing was performed with the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 platform and the Xpert Xpress SARS-CoV-2 assay on the Cepheid Infinity platform. The Xpert Xpress assay gave 2 CT values for the E and N2 targets on positive samples.19 For this assay to indicate a positive specimen, amplification by RT-PCR of the N2 target or both the N2 and E target is required. The Xpert Xpress assay results as presumptive positive if only the E target amplified. This assay counts a maximum of 45 cycles. The Abbott RealTime SARS-CoV-2 assay gave 1 CN derived from the RNA-dependent RNA polymerase and N targets on positive samples.20 The Abbott assay on the m2000 counts a maximum of 37 cycles. The CT/CN value is the number of cycles required by RT-PCR for the fluorescence signal to cross a threshold value exceeding background level.19,20
Samples that are negative for COVID-19 by RT-PCR do not produce a CT/CN value. Although both instruments were used for RT-PCR, the precise CT/CN values are not interchangeable and CT/CN observations over time between the 2 instruments during the disease course would be based on CT/CN value movement (general upward or downward trend) rather than absolute CT/CN differences. Both assays have been approved by emergency use authorization as qualitative tests for the presence/absence of COVID-19. Although the CT/CN value is available to laboratory staff after test completion, the CT/CN value is not reported routinely in the patient health record. All veteran patients identified on the initial review from March 28, 2020, to April 24, 2020, had all serial COVID-19 testing recorded until November 10, 2020. The CN values at the limit of detection (LOD) for the Abbott m2000 platform from the initial validation study were reviewed for reference.21
Results
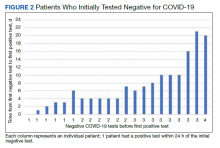
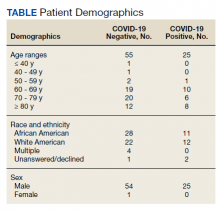
Of 80 patients, 25 (31%) were COVID-19 positive over the course of testing. The study population had a mean age of 73.5 years; 92% were aged > 60 years. The group was predominantly male (79 male vs 1 female). Among the 77 patients with a stated ethnicity, 39 (51%) were African American. In comparison, 43% of residents in Philadelphia County are African American (Table).22,23 Additionally, a previously published total COVID-19 tested population by ethnicity at the same regional VAMC revealed 46.8% of tested veteran patients were African American. 24 Three patients had no stated ethnicity. Among those who tested positive, 11 were African American patients, 12 were White patients, and 2 had no stated ethnicity. Four patients tested positive on their first test. The other 21 patients were positive on repeat testing. Interestingly, 6 patients had 1 initial negative test before a positive test, 6 patients had 2, 8 patients had 3, and 1 patient had 4 initial negative tests before a positive test result. Among the 25 positive patients, 22 were either positive within 10 days of the initial negative test result or initially positive (Figure 2). Three patients who tested positive after 10 days did so at 16, 20, and 21 days after the initial negative test result. Among the 25 positive patients, 23 had initial and serial testing from both the Abbott and Xpert Xpress assays. The remaining 2 positive patients had initial and serial testing from the Abbott assay exclusively.
Only positive COVID-19 results by RTPCR produced a CT/CN value. After disease resolution with a negative test, no CT/CN value was produced with the negative test result on either testing platform. Because repeat testing after the initial positive result took place no sooner than 10 days, we observed that the CT/CN value increased after the initial positive result until the disease resolved, and a negative result was obtained (eAppendix 1, available online at doi:10.12788/fp.0276). A t test comparing the initial CT/CN value to the value more than 10 days after the initial positive showed the CT/CN was statistically significantly higher (P < .05).
Prompt repeat testing after the initial test can show a decrease in the CT/CN value because of increasing viral load before the expected increase until disease resolution if the initial test caught the infection early. Twelve patients had a negative test result between 2 serial positive results. These negative test results occurred later, near the end of the disease course. Among the 12 patients with this positive-negativepositive CT/CN pattern, 7 were symptomatic and no longer had documented symptoms or hospitalization around the time of this positive-negative-positive pattern. Four of these individuals were asymptomatic during the entire infection course. One of the 12 patients with this pattern expired with the negative result occurring on day 27 of the disease in the context of rising CT/CN. One of these 12 patients only had a presumptive positive test result on the Cepheid because it detected only the E target with a CT value of 38.7. In 1 of the 12 patients, the negative test result occurred between 2 positive test results with CT/CN values < 20 (12.05 and 19.05 for the positive tests before and after the negative result, respectively). When the initial CT/CN values was separated based on ethnicity, the average CT/CN value for African Americans (23.3) was higher than for other ethnicities (19.9), although it did not reach statistical significance (P = .35).