Nontuberculous Mycobacterial Pulmonary Disease
Mycobacterium abscessus Complex
Case Patient 2
A 64-year-old man who is an ex-smoker presents with chronic cough, mild shortness of breath on exertion, low-grade fever, and unintentional weight loss of 10 lb. Physical exam is unremarkable. The patient was diagnosed with immunoglobulin deficiency (low IgM and low IgG4) in 2002, and has been on replacement therapy since then. He also has had multiple episodes of NTM infection, with MAC and M. kansasii infections diagnosed in 2012-2014, which required 18 months of multi-drug antibiotic treatment that resulted in sputum conversion. Pulmonary function testing done on this visit in 2017 shows mild obstructive impairment.
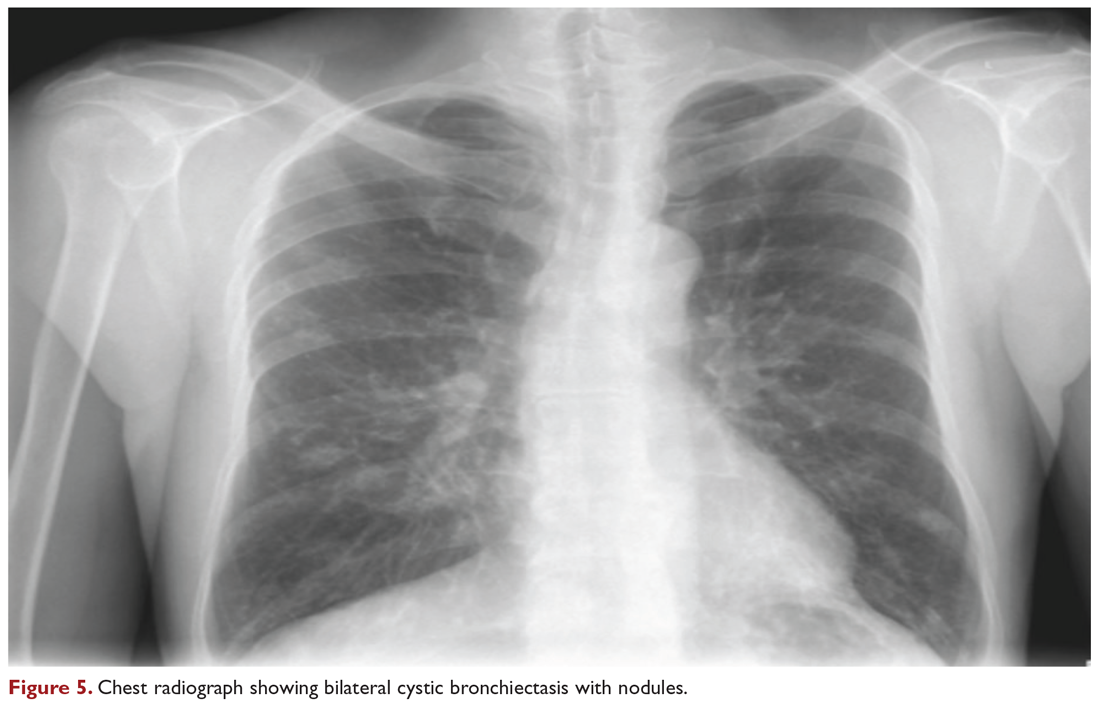
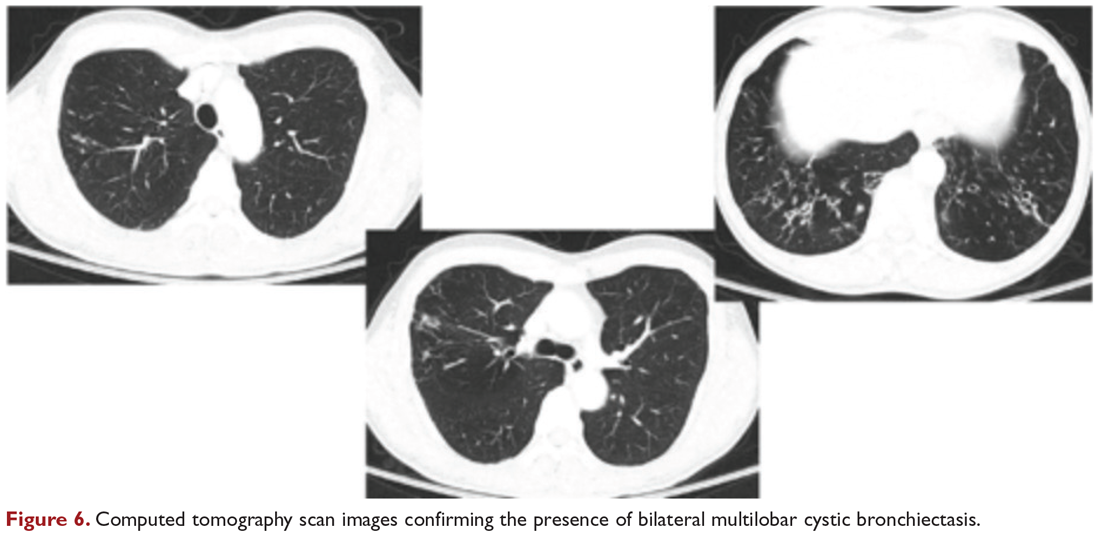
Chest radiograph and CT scan show bilateral bronchiectasis (Figure 5 and Figure 6).
The results of serial sputum microbiology testing performed over the course of 6 months are outlined below:
- 5/2017 (bronchoalveolar lavage): 2+; M. abscessus
- 9/2017 × 2: smear (–); group IV RGM
- 11/2017: smear (–); M. abscessus (> 50 CFU)
- 12/2017: smear (–); M. abscessus (> 50 CFU)
What are the clinical considerations in this patient with multiple NTM infections?
M. abscessus complex was originally described in soft tissue abscesses and skin infections possibly resulting from soil or water contamination. Subspeciation of M. abscessus complex during laboratory testing is critical to facilitate selection of a specific therapeutic approach; treatment decisions are impacted by the presence of an active erm gene and in vitro macrolide sensitivity, which differ between subspecies. The most acceptable classification outlines 3 species in the M. abscessus complex: Mycobacterium abscessus subsp abscessus, Mycobacterium abscessus subsp bolletii (both with an active erm gene responsible for macrolide resistance), and Mycobacterium abscessus subsp massiliense (with an inactive erm gene and therefore susceptible to macrolides).44
RGM typically manifest in skin, soft tissue, and bone, and can cause soft tissue, surgical wound, and catheter-related infections. Although the role of RGM as pulmonary pathogens is unclear, underlying diseases associated with RGM include previously treated mycobacterial disease, coexistent pulmonary diseases with or without MAC, cystic fibrosis, malignancies, and gastroesophageal disorders. M. abscessus is the third most commonly identified respiratory NTM and accounts for the majority (80%) of RGM respiratory isolates. Other NTM reported to cause both lung disease and skin, bone, and joint infections include Mycobacterium simiae, Mycobacterium xenopi, and Mycobacterium malmoense. Ocular granulomatous diseases, such as chorioretinitis and keratitis, have been reported with both RGM and Runyon group III SGM, such as MAC or M. szulgai, following trauma or refractive surgery. These can mimic fungal, herpetic, or amebic keratitis. The pulmonary syndromes associated with multiple culture positivity are seen in elderly women with bronchiectasis or cavitary lung disease and/or associated with gastrointestinal symptoms of acid reflux, with or without achalasia and concomitant lipoid interstitial pneumonia.45
Generally, pulmonary disease progresses slowly, but lung disease attributed to RGM can result in respiratory failure. Thus, RGM should be recognized as a possible cause of chronic mycobacterial lung disease, especially in immunocompromised patients, and respiratory isolates should be assessed carefully. Identification and drug susceptibility testing are essential before initiation of treatment for RGM.
What is the approach to management of M. abscessus pulmonary disease in a patient without cystic fibrosis?
The management of M. abscessus pulmonary infection as a subset of RGM requires a considered step-wise approach. The criteria for diagnosis and threshold for starting treatment are the same as those used in the management of MAC pulmonary disease,11 but the treatment of M. abscessus pulmonary infection is more complex and has lower rates of success and cure. Also, antibiotic treatment presents challenges related to rapid identification of the causative organism, nomenclature, resistance patterns, and tolerance of treatment and side effects. If a source such as catheter, access port, or any surgical site is identified, prompt removal and clearance of the infected site are strongly advised
In the absence of any controlled clinical trials, treatment of RGM is based on in vitro susceptibility testing and expert opinion. As in MAC pulmonary disease, macrolides are the mainstay of treatment, with an induction phase of intravenous antibiotics. Treatment may include a combination of injectable aminoglycosides, imipenem, or cefoxitin and oral drugs such as a macrolide (eg, clarithromycin, azithromycin), doxycycline, fluoroquinolones, trimethoprim/sulfamethoxazole, or linezolid. While antibiotic treatment of M. abscessus pulmonary disease is based on in vitro sensitivity pattern to a greater degree than is treatment of MAC pulmonary disease, this approach has significant practical limitations and hence variable applicability. The final choice of antibiotics is best based on the extended susceptibility results, if available. The presence of an active erm gene on a prolonged growth specimen in M. abscessus subsp abscessus and M. abscessus subsp bolletii precludes the use of a macrolide. In such cases, amikacin, especially in an intravenous form, is the mainstay of treatment based on MIC. Recently, there has been a resurgence in interest in the use of clofazimine in combination with amikacin when treatment is not successful in patients with M. abscessus subsp abscessus or M. bolletii with an active erm gene.45,46 When localized abscess formation is noted, surgery may be the best option, with emphasis on removal of implants and catheters if implicated in RGM infection.
Attention must also be given to confounding pulmonary and associated comorbidities. This includes management of bronchiectasis with appropriately aggressive airway clearance techniques; anti-reflux measures for prevention of micro-aspiration; and management of other comorbid pulmonary conditions, such as chronic obstructive pulmonary disease, pulmonary fibrosis, and sarcoidosis, if applicable. These interventions play a critical role in clearing the M. abscessus infection, preventing progression of disease, and reducing morbidity. The role of immunomodulatory therapy needs to be considered on a regular, ongoing basis. Identification of genetic factors and correction of immune deficiencies may help in managing the infection.
Case Patient 2 Conclusion
The treatment regimen adopted in this case includes a 3-month course of daily intravenous amikacin and imipenem with oral azithromycin, followed by a continuation phase of azithromycin with clofazimine and linezolid. Airway clearance techniques such as Vest/Acapella/CPT are intensified and monthly intravenous immunoglobulin therapy is continued. The patient responds to treatment, with resolution of his clinical symptoms and reduction in the colony count of M. abscessus in the sputum.
Summary
NTM are ubiquitous in the environment, and NTM infection has variable manifestations, especially in patients with no recognizable immune impairments. Underlying comorbid conditions with bronchiectasis complicate its management. Treatment strategies must be individualized based on degree of involvement, associated comorbidities, immune deficiencies, goals of therapy, outcome-based risk-benefit ratio assessment, and patient engagement and expectations. In diffuse pulmonary disease, drug treatment remains difficult due to poor match of in vitro and in vivo culture sensitivity, side effects of medications, and high failure rates. When a localized resectable foci of infection is identified, especially in RGM disease, surgical treatment may be the best approach in selected patients, but it must be performed in centers with expertise and experience in this field.






