UPDATE ON CERVICAL DISEASE
Will co-testing further reduce your patients’ risk of cervical Ca, compared with current Pap screening alone? How should you care for a woman who has a Pap–/HPV+ result? Here are answers, illuminated by new data.
IN THIS ARTICLE
Data to support the application to the FDA for approval of the cobas 4800 was provided by the ATHENA Trial, the largest (47,208 women) cervical screening trial of US women to assess the performance of HPV DNA testing with individual genotyping for HPV 16, 18, compared with the Pap.
Wright and co-workers. These investigators evaluated the subset of 32,260 women 30 years and older who had negative cytology. The overall prevalence of Pap–/HPV+ was 6.7%. Just over one quarter of the Pap–/HPV+ women (1.5% of the subset population) were positive for HPV 16 or 18, or both.
As has been shown in other studies, the overall prevalence of HPV declined with age, as did the prevalence of HPV 16, 18. The estimated absolute risk of CIN 3+ at colposcopy for women who were Pap–/HPV+ was only 4.1%, but their relative risk—that is, compared with women in whom both tests were negative—was 14.4%. The absolute risk for CIN 3+ increased to 9.8% for Pap-negative women who tested positive for HPV 16 or 18, or both, and to 11.7% in women who tested positive for HPV 16 only.
Ongoing work by Castle and colleagues. In a seminal 2007 article on risk management, Castle and colleagues proposed that women who had an absolute risk of CIN 3+ of ≥10% across 2–3 years of follow-up should have colposcopy.4 These data, therefore, clearly support the recommendation of ASCCP that Pap–/HPV 16+ and (or) HPV 18+ women should be referred for colposcopy (FIGURE 3).
FIGURE 3 Managing risk of CIN 3+ across 2 years of follow-up
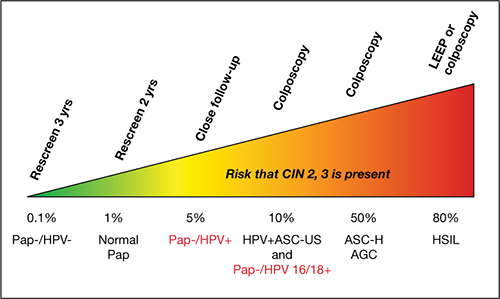
This stratagem is based on 2006 ASCCP Consensus Guidelines, 2010 ACOG guidelines, and Castle and colleagues’ proposal in their 2007 article, “Risk assessment to guide the prevention of cervical cancer.”4
Figure courtesy of Thomas C. Wright, MD.
Now, in another review of the ATHENA results, expanded to a subset of women 25 years and older, Castle and co-workers reported that triage of Pap–/HPV+ women by testing for HPV 16, 18 detected 72% of all CIN 3+ that was missed by cytology alone. Because only 18% of Pap–/HPV+ women were positive for HPV 16, 18, the majority (i.e., 82% who were ≥25 years and 78% who were ≥30 years) could be reassured that their risk was sufficiently low that it could be best managed by repeating co-testing in 1 year. This avoids delaying the diagnosis of nearly three quarters of the Pap–/HPV+ women who have CIN 3+, without overburdening colposcopy services.
The ATHENA trial is ongoing; eventually, results from 3 years of follow-up of these women will be evaluated. The findings should add important information about the total burden of cervical precancer detected over a longer period after a Pap–/HPV+ result, with or without testing positive for HPV 16, 18.
Among your patients who are Pap–/HPV+, testing for HPV 16, 18 allows you to identify most of those who are at highest risk of CIN 3+ (i.e., HPV 16+ or HPV 18+, or both)—and who will, therefore, be most likely to benefit from immediate colposcopy.
We want to hear from you! Tell us what you think.







