Fatigue, arthalgia, amenorrhea—Dx?
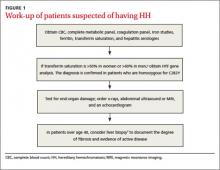
Additional testing for patients in whom you suspect HH includes:
• a complete blood count, metabolic panel, and coagulation panel
• hepatitis serologies
• imaging (abdominal ultrasound, skeletal radiographs, echocardiogram, abdominal magnetic resonance imaging [MRI])
• a liver biopsy with iron staining and quantitative iron measurements.
The gold standard. Performing a liver biopsy to measure hepatic iron concentration by staining is considered the gold standard test for HH.8 But since genetic testing has become more readily available, liver biopsies aren’t widely used to confirm the diagnosis.8 The diagnosis of HH usually is confirmed by molecular testing for the C282Y and H63D mutations. Liver biopsy may be recommended to document the degree of fibrosis in all homozygotes over age 40 with elevated serum transaminase levels, clinical evidence of liver disease, or a serum ferritin level >1000 mcg/L.7
Phlebotomy helps lower iron levels
Treatment should not be delayed until symptoms develop.3 The mainstay of therapy is phlebotomy.9 If phlebotomy is started before the onset of organ damage, patients can anticipate a normal lifespan.9 Without treatment death may occur from cirrhosis, hepatocellular carcinoma, or cardiomyopathy.
Removal of 1 unit of red blood cells (450-500 mL) results in the loss of approximately 200 mg of iron. Serum ferritin level testing is the most reliable and least expensive method to monitor therapy.9 Iron depletion is complete when the serum ferritin level is 10 to 20 g/L, when the hemoglobin concentration is <11 g/dL, or the hematocrit is <33% for >3 weeks. HH patients need to undergo lifelong phlebotomy to maintain a serum ferritin level <50 g/L. Encourage patients to take in an adequate amount of dietary protein, vitamin B12, and folate to support the accelerated level of erythropoiesis that occurs during therapy.9
Chelation therapy is reserved for patients with advanced disease (eg, those with organ damage) or those who do not respond to phlebotomy.10 Deferoxamine given intravenously (IV) or subcutaneously has been the standard chelation agent. It’s usually administered by continuous subcutaneous infusion using a battery-operated pump at a dose of 40 mg/kg/d for 8 to 12 hours nightly for 5 to 7 nights weekly. A dose of approximately 2 g per 24 hours usually achieves maximal urinary iron excretion.
The use of deferoxamine therapy is limited by cost as well as the need for parenteral therapy, discomfort, inconvenience, and neurotoxicity.5 The US Food and Drug Administration recently approved an oral ironchelating agent, deferasirox, for the treatment of secondary iron overload due to ineffective erythropoiesis. Studies are ongoing to evaluate its potential use in HH.5,9
Our patient’s outcome
Our patient declined liver biopsy and her sisters declined HFE genotyping. Our patient did, however, complete 7 phlebotomies over 4 months. Two months later, she reported shortness of breath during exertion, leg swelling, and palpitations. A chest x-ray revealed a right-sided pleural effusion and an electrocardiogram showed atrial fibrillation with rapid ventricular response. Our patient was admitted for telemetry monitoring and started on diltiazem IV. Echocardiogram showed a restrictive cardiomyopathy, with an ejection fraction of 15% (normal range >55%).
Six weeks later, her ejection fraction decreased to 10%. An MRI of her abdomen showed iron deposition in her liver, pancreas, and lymph nodes (FIGURE 2). She was started on deferoxamine IV and transferred to the coronary care unit for 3 weeks. She was discharged with a diagnosis of class IV heart failure and admitted 2 weeks later for exacerbation of heart failure symptoms. She did not want to pursue a heart transplant. Her condition deteriorated and she expired after a fatal cardiac arrhythmia.
THE TAKEAWAY
Patients with abnormal iron studies and those with evidence of liver disease should be evaluated for HH5 (strength of recommendation [SOR]: A). Fasting serum transferrin saturation and serum ferritin concentration are recommended as initial tests for HH11 (SOR C). Liver biopsy is the gold standard for diagnosis of HH, but the diagnosis usually is confirmed by genetic testing8 (SOR C). Phlebotomy is the mainstay of therapy9 (SOR B). Chelation therapy is reserved for patients with advanced disease or for those who do not respond to phlebotomy10 (SOR C).
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series