Evaluation and treatment of the patient with allergic rhinitis
Probiotics. Epidemiologic studies suggest that the increase in atopic disease may be related to a clean environment and widespread use of antibiotics in Western countries. The environment may deprive fetal and infant immune systems of bacterial antigens that stimulate type 1 T-helper (Th1) cells.8 In light of this theory, Finnish researchers randomly assigned 159 pregnant women with a family history of atopy to receive capsules of Lactobacillus GG (a potentially beneficial bacteria or “probiotic”) or placebo, beginning 2 to 4 weeks prior to delivery and continuing 6 months postpartum. Infants were followed for 2 years. Frequency of atopic dermatitis was reduced by 50% among those infants whose mothers received Lactobacillus.40 Further study of this association in allergic rhinitis would be beneficial.
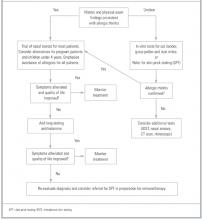
Treatment recommendations.Table 2 summarizes treatment-related evidence in the management of allergic rhinitis, and the Figure illustrates a proposed treatment algorithm. Another algorithm by the European Academy of Allergy and Clinical Immunology recommends initial therapy with oral or nasal antihistamines for mild disease, nasal corticosteroids for moderate disease, and both for severe disease.10 This was a consensus opinion. Of the 2 most commonly used medications for allergy, nasal steroids are favored over antihistamines for overall safety, tolerability, effectiveness, and simplicity in all cases. In one study of 61 adults, patients were randomized to receive either a nasal steroid or an antihistamine as initial therapy, with the other agent reserved as “back-up.” After 6 weeks, 86% of patients started on an antihistamine had added their steroid back-up, while 51% of the group started on a steroid remained on that agent alone.41 Starting all patients on both an antihistamine and a nasal steroid is inappropriate.
TABLE 2
Evidence to support treatment recommendations
| Strength of recommendation | Treatment | Comment |
|---|---|---|
| A | Immunotherapy | Can have long lasting clinical benefit. |
| A | Intranasal Consistently | superior to antihistamines corticosteroids in head to head trials. Not clear if all steroids are equally effective. |
| A | Antihistamines | Effective, but inferior to intranasal steroids in most clinical outcomes. |
| A | Cromolyn sodium | Intranasal steroids superior in all clinical outcomes. |
| B | Decongestants | Less effective than antihistamines in direct comparisons, many trials involve combination products. |
| D | Probiotics | Larger trials needed, limited evidence. |
| D | Herbal medications (licorice, gingko, ginseng) | Limited evidence. |
FIGUREA guide to evaluation and treatment of allergic rhinitis
Prognosis
The long-term prognosis for allergic rhinitis is excellent. For most patients, the illness is primarily a nuisance with no significant morbidity. However, for patients whose rhinitis is moderate to severe and poorly controlled, there can be significant complications. These complications include asthma, sinusitis, otitis media, nasal polyposis, respiratory infections, and orthodontic malocclusions.42 In one study of 605 children with allergic rhinitis, 21% had chronic otitis media with effusion (OME). Conversely, in another study of 259 children with OME, 50% had allergic rhinitis.43 Even among patients without asthma, 20% to 30% will have bronchial hyper-responsiveness. Additionally, poorly controlled allergic rhinitis can contribute to sleep loss, daytime fatigue, and learning impairment.44
Potential complications related to long-term treatment in children remain controversial. In a 1998 study of intranasal beclomethasone, children receiving the study medication grew an average of only 5 centimeters (cm) in 1 year, compared with an average of 5.9 cm in the placebo group.45 However, a similar study done 2 years later with intranasal mometa-sone showed no evidence of growth suppression.45 Further studies are needed before the true impact of intranasal steroids on children can be determined.
· Acknowledgments ·
The opinions and assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the U.S. Army or the U.S. Department of Defense. The author wishes to thank Kathleen Conner, JD, for her assistance in the preparation of the manuscript.