Inpatient antibiotic resistance: Everyone’s problem
Whether you’re seeing patients in the hospital or after discharge, awareness of their inpatient antibiotic exposure and support of antibiotic stewardship practices are key to combating antibiotic resistance.
PRACTICE RECOMMENDATIONS
› Consider alternatives to vancomycin for health care-associated methicillin-resistant Staphylococcus aureus isolates with a vancomycin minimum inhibitory concentration >2 mcg/mL or in the setting of poor clinical response. A
› Identify colonization vs infection with vancomycin-resistant enterococci (VRE) in the gastrointestinal tract following antibiotic exposure to minimize inappropriate antibiotic prescribing for VRE. C
› Use carbapenems as first-line treatment for severe infections caused by Enterobacteriaceae-producing extended-spectrum beta-lactamases. C
› Treat invasive carbapenem-resistant Enterobacteriaceae infections with combination therapy; site of infection, susceptibility patterns, and patient-specific factors should guide antibiotic selection. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
,Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting. 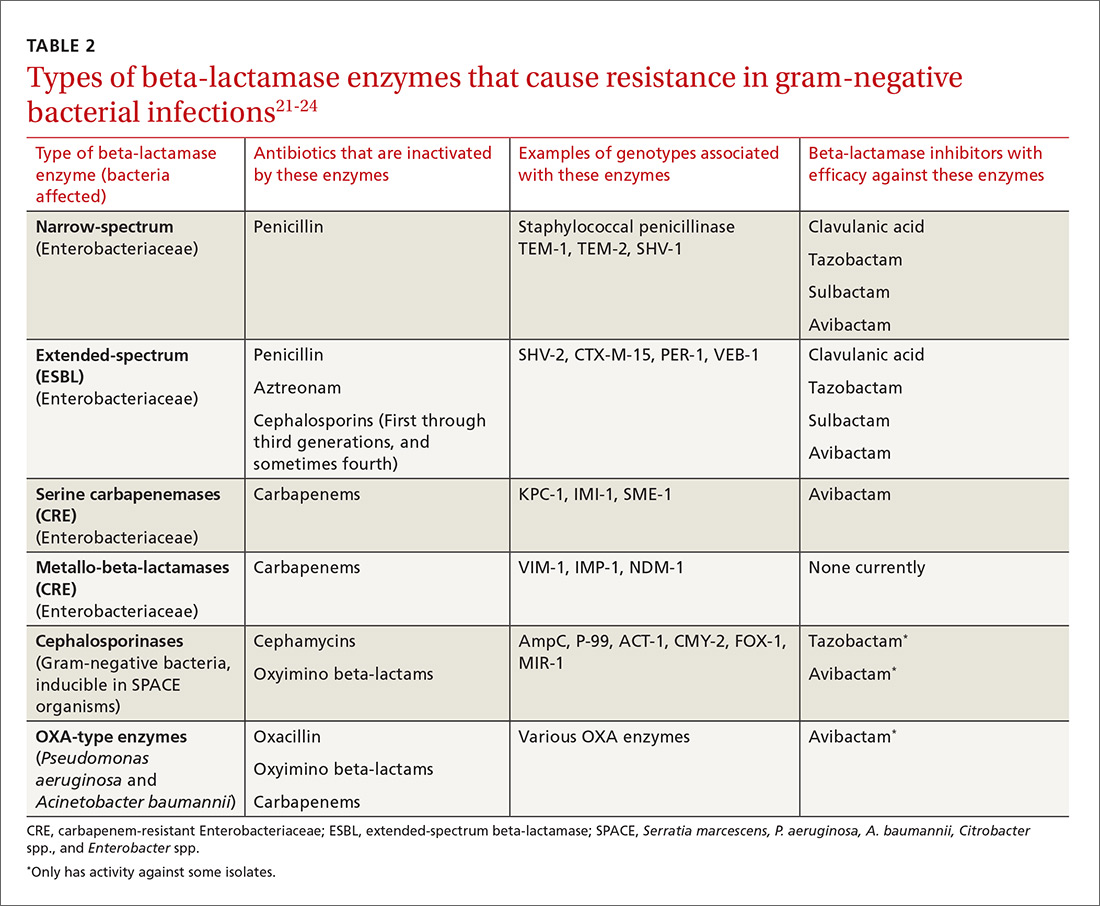
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22
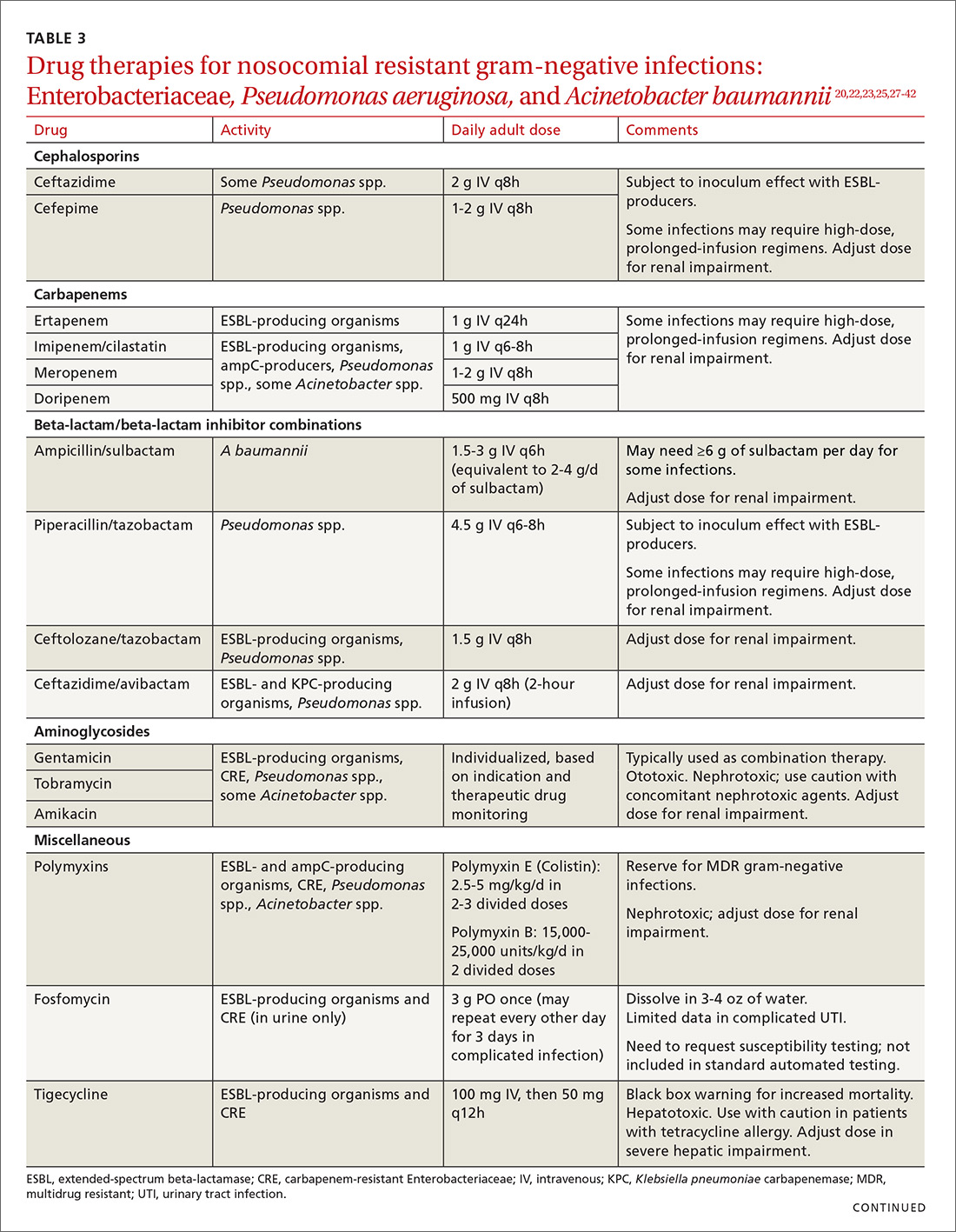
Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35






