Antibiotic stewardship: The FP’s role
Drug resistance is an expanding problem in outpatient settings. The text and tables that follow can help you fight it by adhering to optimal prescribing guidelines.
PRACTICE RECOMMENDATIONS
› Manage uncomplicated cutaneous abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus with incision and drainage alone. A
› Treat upper respiratory infections associated with drug-resistant Streptococcus pneumoniae with high-dose amoxicillin, which has been found to overcome penicillin resistance. A
› Administer dual therapy with ceftriaxone and azithromycin to patients with gonococcal infections. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
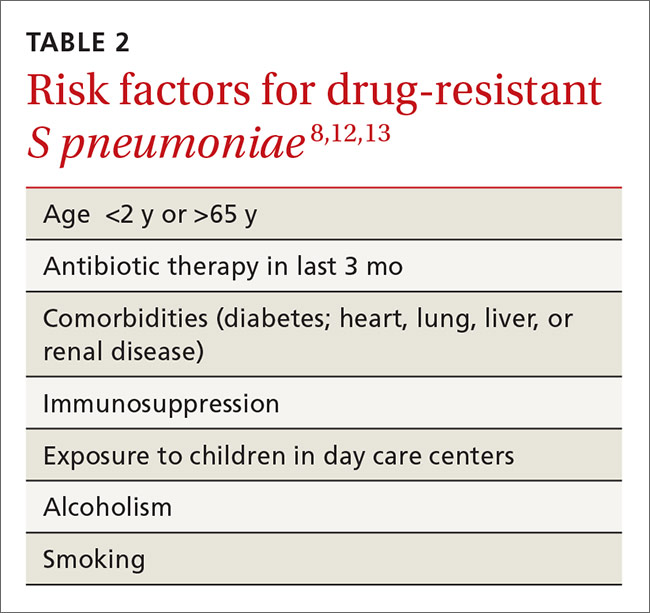
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13
Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19
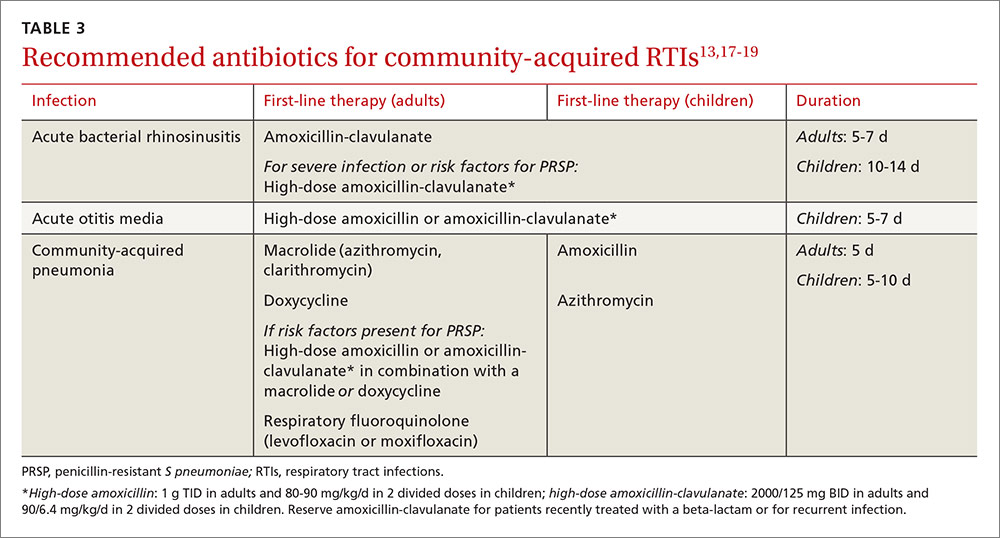
High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1






