Updates in Multiple Sclerosis Imaging
Background: Multiple sclerosis (MS), one of the most common causes of neurological disability in young adults, is a chronic central nervous system disease characterized by immune-mediated inflammation, demyelination, and neurodegeneration. MS may be difficult to diagnose due to its protean neurological manifestations and the multitude of other neurologic conditions that can produce white matter lesions similar to MS demyelinating lesions. The wide clinical variability of the disease makes it challenging to provide an accurate prognosis in an individual with MS.
Observations: Magnetic resonance imaging (MRI) biomarkers such as T2-lesions, chronic black holes, atrophy, paramagnetic rim lesions (PRL), and the central vein sign (CVS), may assist clinicians with the diagnosis and prognostication of MS. Underscoring their importance, PRL and CVS will be incorporated into the 2024 iteration of the McDonald Criteria for the diagnosis of MS. Quantitative MRI techniques, utilized in translational research, can quantify the degree of microstructural injury and guide the development of future therapies. This review discusses the impact, recent advances, and limitations of imaging biomarkers and quantitative MRI techniques with regard to routine MS clinical care and translational research.
Conclusions: Clinicians caring for people with MS should have a basic understanding of imaging biomarkers and their implications for routine clinical care.
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
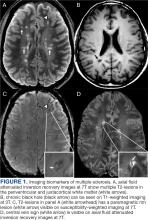
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.