Systemic Therapy in Metastatic Melanoma
Melanoma is the most aggressive form of skin cancer, contributing to about 76,000 new cases and more than 9,000 deaths in 2014.1 Depending on the stage of the disease, 5-year melanoma survival can range from 15% to 97%. Patients with local and distant metastases have a 5-year survival of about 60% and 15%, respectively.2
The incidence of melanoma is rising, partly because of the increasing number of skin biopsies being performed.3 If melanoma is diagnosed early, surgical excision is the treatment of choice. In patients with oligometastatic disease (cancer that has spread, but only to 1 or a small number of sites), complete surgical excision of the metastases may provide prolonged overall survival (OS) and delay the need to use systemic therapy.4
Recently, many new drug therapies have shown promising results in clinical trials, which may improve the prognosis of metastatic disease. This article reviews currently available systemic treatment options for the management of metastatic melanoma, the role of cytotoxic chemotherapy and interleukin-2 (IL-2), and the latest therapies available, including immune checkpoint inhibitors.
Cytotoxic Chemotherapy and Interleukin-2
Cytotoxic chemotherapy does not have an established role in the initial treatment of metastatic melanoma. Currently, cytotoxic chemotherapy is used in patients who have not responded to immunotherapy or molecular targeted therapy. The most commonly used drugs include dacarbazine and its prodrug, temozolomide. Several studies have failed to demonstrate a survival benefit using a single-agent chemotherapy with either dacarbazine or temozolomide.5,6
Other agents used in metastatic melanoma include nitrosoureas (fotemustine), platinum compounds (cisplatin, carboplatin), vinca alkaloids (vincristine),
and taxanes (paclitaxel). None of these agents provide a survival benefit, but an objective response may be seen in a minority of cases. Combination chemotherapy regimens have not shown an advantage over singleagent dacarbazine or temozolomide.7,8
High-dose IL-2 has been used in cases of metastatic melanoma with good performance status (PS) and organ function. Studies have shown a complete response rate of 3% to 7% and a prolonged disease-free survival in a minority of patients.9-11 The use of highdose IL-2, however, is limited by the high incidence of adverse effects (AEs), which include bacterial sepsis, pulmonary edema, arrhythmias, fever, and on some occasions, death due to complications.10 The use of IL-2 requires admission of the patient to a specialized unit for AE monitoring and management. Because of its ability to “cure” a minority of patients, a role still exists for IL-2 therapy in the treatment of younger, healthy patients with no evidence of organ dysfunction at baseline.
Immune Checkpoint Inhibitors
Checkpoint inhibitors are a class of drugs that unmask the immune system to fight against cancer cells. This class of drugs has shown significant activity and survival advantage in recent phase 2 and 3 trials. The class includes the anticytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody ipilimumab and monoclonal antibodies targeting the programmed death 1 protein (PD-1) or its ligand (PD-L1).
Anti-CTLA-4 Antibodies: Ipilimumab
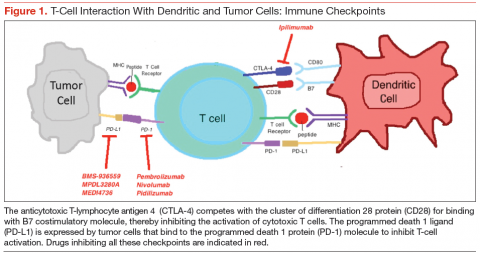
Cytotoxic T-lymphocyte antigen 4 is the antigen responsible for inhibition of cytotoxic T-cell-mediated immunity against foreign antigens presented by the antigen presenting cells (APCs). The APCs cause activation of the T cells when peptide fragments of intracellular proteins are presented in combination with mixed histocompatibility complex molecules. This step requires interaction of a costimulatory molecule (B7) on the APCs with a cluster of differentiation 28 protein (CD28) receptor located on T cells. CTLA-4 competes with CD28 to bind with the B7 molecule, thereby inhibiting the activation of the cytotoxic T cells (Figure 1). This pathway is thought to help with development of tolerance to host tissue antigens. Ipilimumab is a human monoclonal antibody that inhibits this CTLA-4 molecule and facilitates T-cell mediated antitumor activity.12 By blocking the CTLA-4 molecule, ipilimumab also mediates its autoimmune AEs on the host tissues.
Hodi and colleagues conducted a phase 3 trial of ipilimumab, including 676 patients who progressed after prior treatment for stage III or IV melanoma, and found that median OS was significantly better in the ipilimumab groups: 10 months in the ipilimumab plus gp100 peptide vaccine group vs 6.4 months in the gp100 vaccine alone group; 10.1 months in the ipilimumab alone group vs 6.4 months in the gp100 vaccine alone group.13 In another phase 3 trial comparing ipilimumab plus dacarbazine to dacarbazine alone, the ipilimumab group had a significantly improved OS (11.2 months vs 9.1 months).1 Survival rates with ipilimumab were prolonged for up to 3 years compared with the dacarbazine plus placebo group. However, the combination was associated with increased incidence of hepatotoxicity, thereby limiting its use.
A long-term survival analysis of 10 prospective and 2 retrospective studies of ipilimumab showed a median OS of 11.4 months and a long-term survival that began at 3 years with a plateau at 10 years of 21%, which was independent of prior therapy or ipilimumab dose.14 The immune-related AEs of ipilimumab are secondary to its activity against the host antigens and include dermatitis, enterocolitis, hepatitis, and endocrinopathies.15
A recent phase 2 trial studied the combination of ipilimumab with granulocyte-macrophage colonystimulating factor in 245 patients with stage III and IV melanoma. Median OS after 13 months was significantly higher with the combination compared with ipilimumab alone. The 1-year survival rate was 69% with
the combination and 53% with ipilimumab alone. There was no difference in the overall response rate (ORR) or progression-free survival (PFS) between the 2 groups. However, the AEs were significantly reduced with the combination (45% vs 58%).16 The dose of ipilimumab used in the trial was higher than the approved dose, making it difficult to apply the results in practice without further studies on the combination.
Anti-PD-1 Antibodies
Programmed death 1 ligands (PD-L1 and PD-L2) are expressed by tumor or stromal cells to inhibit the T-cell mediated antitumor activity. These ligands bind to the PD-1 protein on the surface of activated T cells to mediate their immunosuppressive effects. Interruption of this interaction by either anti-PD-1 antibodies or anti-PD-L1 antibodies facilitates tumor cell killing by activated T cells.17
Pembrozilumab and nivolumab are the 2 anti-PD-1 monoclonal antibodies that have been approved for treatment of metastatic melanoma. In a phase 1 trial
of pembrolizumab, 411 patients with advanced melanoma (consisting of both ipilimumab-naïve [IPI-N] and ipilimumab-treated [IPI-T] patients), ORR was 40% in IPI-N and 28% in IPI-T patients with a 1-year OS of 71% in all patients. Median PFS was 24 weeks in IPI-N and 23 weeks in IPI-T pts.18 There was no difference in outcomes and safety profiles across the various dosing regimens.18,19 Of note, pembrolizumab had antitumor activity irrespective of the PS, lactate dehydrogenase levels, BRAF (B-Raf proto-oncogene, serine/threonine kinase) gene mutation, metastatic stage, and number and type of prior therapy. In a subgroup analysis, 173 patients who had progression after treatment with ipilimumab were randomly assigned to pembrolizumab 2 mg/kg every 3 weeks (q3w) or 10 mg/kg q3w dosing regimens. Both groups had no significant difference in the ORR (26% in both) and safety profiles.20
In the 2012 KEYNOTE-002 clinical trial, a randomized phase 2 trial involving 540 patients with ipilimumab-refractory advanced melanoma, patients were randomized 1:1:1 to pembrolizumab 2 mg/kg or 10 mg/kg q3w or investigator-choice chemotherapy (control arm consisting of carboplatin plus paclitaxel, carboplatin, paclitaxel, dacarbazine, or temozolomide). The 6-month PFS was significantly improved with pembrolizumab (34% and 38% for pembrolizumab 2 mg/kg and 10 mg/kg, respectively) compared with 16% with chemotherapy. The ORR was significantly better with pembrolizumab (21% at 2 mg/kg, 25% at 10 mg/kg) compared with the control arm (4%).21 These findings led to the approval of pembrolizumab by the FDA for treatment of patients with advanced melanoma who have progressed on ipilimumab. Pembrolizumab is generally well tolerated. The most common AEs include fatigue, pruritus, and rash.
Nivolumab was studied in a recent phase 1 trial in which 107 patients with previously treated advanced melanoma were treated with escalated doses every
2 weeks.22 The 2-year and 3-year OS rates were 48% and 41%, respectively. Objective responses were seen in 32% of the patients. The median response duration was 23 months.23
The first phase 3 trial was conducted in 418 patients with previously untreated metastatic melanoma BRAF mutation. Patients were randomized to receive either nivolumab or dacarbazine. The PFS and OS were significantly better with nivolumab compared with dacarbazine (PFS 5.1 months vs 2.2 months; OS 73% vs 42% at 1 year).24 The AE profile of nivolumab is similar to pembrolizumab and includes lung, skin, endocrine, renal, and gastrointestinal tract toxicities.
Preliminary results of another phase 3 trial were presented at the European Society of Medical Oncology 2014 meeting. Patients with previously treated metastatic melanoma (ipilimumab or BRAF inhibitor) were randomized in a 2:1 ratio to receive either nivolumab or investigators’ choice chemotherapy (dacarbazine or carboplatin plus paclitaxel). The ORR was significantly better with nivolumab (32% vs 11%), and 95% of patients were still responding after 6 months. The nivolumab group showed a complete remission in 3% of the patients with 34% of the responses lasting ≥ 6 months.25 This led to the recent approval of nivolumab for patients with metastatic melanoma with a BRAF mutation who have advanced on ipilimumab. In the phase 3 NCT01844505 trial patients are being randomized to receive ipilimumab, nivolumab, or both.
A newer PD-1 inhibitor, pidilizumab, was studied in a phase 2 trial that included 103 patients with metastatic melanoma, 51% of whom had received therapy with ipilimumab. The ORR in the study group was relatively lower (6%), but the OS at 1 year was 64.5%.26 Further studies are underway to evaluate the role of this drug in metastatic melanoma.
The response with both nivolumab and pembrolizumab is durable as well as sustained, even after discontinuation of therapy. None of the deaths in the aforementioned studies were atributed to drug-related toxicities. As evidenced by current data, these 2 drugs hold a great promise for the management of patients who progress after therapy with anti-CTLA-4 antibodies.
Anti-PD-L1 Antibodies
The anti-PD-L1 monoclonal antibodies work in a similar way to the PD-1 inhibitors and block the interaction between the PD-1 and its ligand, PD-L1. This causes sustained activation of cytotoxic T cells and facilitates their antitumor activity. Two of PD-L1 inhibitors have shown clinical activity against metastatic melanoma.
BMS-936559, the first PD-L1 antibody, is being studied in a phase 1 trial that includes 55 patients with advanced melanoma along with 152 patients with other solid malignancies. Three patients achieved a complete response, and 5 patients had an objective response lasting 1 year. The ORR for melanoma was 17%, with disease stabilization of ≥ 24 weeks in 27% of the patients.27 Common AEs included infusion reactions, diarrhea, fatigue, rash, hypothyroidism, and hepatitis.
The second PD-L1 antibody, MPDL3280A, was studied in a phase 1 trial of 45 patients with metastatic melanoma. An ORR of 29% was observed, along with a 24-week PFS of 43%.28 Commonly noted AEs included hyperglycemia and elevated liver aminotransferases.
A newer PD-L1 inhibitor, MEDI4736, is being studied for advanced malignancies in 8 patients with melanoma. In preliminary analysis, MEDI4736 demonstrated a partial response in 1 out of 8 melanoma patients with a disease control rate of 46%.29 Although the PD-L1 inhibitors seem promising, more information will help discern their role in the management of metastatic melanoma.
Combined Anti-CTLA-4 Plus Anti-PD-1 Antibody
The combination of ipilimumab and the PD-1 inhibitor nivolumab was tested in a phase 1 trial in which both drugs were used concurrently as well as sequentially in metastatic melanoma.30 The 1- and 2-year OS in patients who were treated concurrently was 82% and 75%, respectively. Complete remission was seen in 17% of the patients, and the responses were seen irrespective of the BRAF mutation status. The responses were durable, and about 64% of the objective responses remain in remission at last follow-up.31 Grade 3 to grade 4 AEs were noted in 53% of the patients, with 11 patients requiring discontinuation of the medications. More studies are required to ascertain the optimum dosage of the combination prior to its approval for use in metastatic melanoma.
Molecular Targeted Therapy
The RAS-RAF–mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway is activated in almost 90% of patients
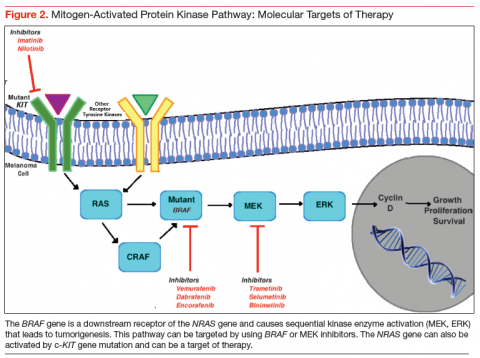
with melanoma.32 This pathway is normally required for the growth and survival of nonmalignant cells. In malignant transformation, mutations and/or overexpression is seen at various levels including KIT, NRAS, BRAF, and the MEK protein. This leads to activation of serine and threonine protein kinases, which lead to uncontrolled cell proliferation and survival.33
Novel therapeutic approaches have tried inhibiting one or more of these pathways for melanoma treatment. The most important mediator of tumorigenesis is BRAF, which is a downstream receptor of NRAS, and is mutated in almost 50% of melanoma cases.34 NRAS mutations are seen in 15% to 20% of cutaneous melanomas.35,36 After its activation, the RAF enzyme—coded by the BRAF gene—causes phosphorylation of the MEK protein, which activates ERK. This ERK activation leads to growth signaling and is the final pathway in several malignancies (Figure 2).37,38
BRAF Inhibitors
BRAF is the first mediator whose inhibition led to clinically significant outcomes in patients with melanoma. The most common BRAF mutation consists of the
substitution of glutamic acid for valine at amino acid 600 (V600E mutation) with majority of the remainder consisting of an alternate substitution (V600V or V600K).34 Vemurafenib and dabrafenib are the 2 BRAF inhibitors that have been shown to improve tumor regression, PFS, and OS considerably, especially in combination with a MEK protein inhibitor. In the phase 3 BRIM-3 trial, the vemurafenib group had a significantly prolonged PFS and OS compared with dacarbazine (13.6 months vs 9.7 months; 6.9 months vs 1.6 months, respectively). It was the first study to show improved survival with vemurafenib in both the V600E and V600K BRAF mutant melanomas.39
Another BRAF inhibitor, dabrafenib, was approved by the FDA for treatment of advanced melanoma with BRAF V600E mutation. It was tested in a phase 3 trial in which it was compared with dacarbazine in patients with advanced melanoma. Median OS in the dabrafenib arm was > 18 months and in dacarbazine arm > 15 months.40 Fifty-seven percent of the patients in dacarbazine arm were crossed over to the dabrafenib arm, thereby confounding the survival data for the former group. Another multicenter, phase 2 trial showed dabrafenib to have activity in melanoma patients with brain metastases, irrespective of previous therapy for the brain metastases.41 The long-term analysis of the BREAK-2 trial, which included 92 patients with metastatic melanoma treated with dabrafenib, showed a median OS of 12.9 months in BRAF V600K group and 13.1 months in BRAF V600E group.42
Adverse effects associated with BRAF inhibition include fatigue, rash, arthralgia, and photosensitivity reactions.43 Dermatologic complications may also include squamous cell carcinoma (SCC) (19%-26%), with keratoacanthoma being the most common subtype.44 These are believed to be likely secondary to the paradoxical activation of the MAPK signaling, since most of these lesions are found to have mutations in the RAS molecule.45 Other specific AEs of dabrafenib include hyperkeratosis (33%) and pyrexia (29%).42
Most patients treated with a BRAF inhibitor eventually have disease progression, likely secondary to reactivation of the MAPK pathway.46,47 This result has led to a heightened interest in combination therapies in an effort to improve outcomes. Combination therapy with ipilimumab and vemurafenib was studied and resulted in a higher incidence of hepatotoxicity (50%).48 However, no hepatotoxicity was seen in a phase 1 trial of combined dabrafenib and ipilimumab.49
Some studies have also suggested that extended BRAF inhibition after progression on a BRAF inhibitor may prolong survival.50,51 The phase 2 trial NCT01983124 is being conducted to evaluate the survival benefit with a combination of vemurafenib and a nitrosourea alkylating agent, fotemustine, in patients who have progressed on vemurafenib alone.
MEK Inhibitors
The inhibition of MEK can halt cell proliferation and induce apoptosis. The phase 3 METRIC trial, which compared the oral MEK inhibitor (trametinib) with chemotherapy, was conducted in 322 patients who had metastatic melanoma with a V600E or V600K BRAF mutation. The PFS and 6-month OS were significantly better with trametinib (4.8 months vs 1.5 months, 81% vs 66%) despite the crossover between the 2 groups.52 The AEs associated with trametinib included rash, diarrhea, and peripheral edema. Another phase 2 trial of trametinib including patients pretreated with a BRAF inhibitor showed no confirmed objective responses, 28% patients with stable disease, and minimal improvement in PFS (2 months). Among patients treated with prior chemotherapy and/or immunotherapy, trametinib showed significant improvement in complete responses, partial responses, stable disease, and the median PFS (2%, 23%, 51%, 4 months, respectively).53
The second MEK inhibitor, binimetinib, was studied in a phase 2 trial of advanced melanoma cases harboring a BRAF V600E or NRAS. Bimetinib demonstrated a PR in 20% cases of both the BRAF and NRAS mutant melanomas. Durable disease control was seen in 43% of the NRAS group and 32% of the BRAF group.54 The AE profile was similar to that seen with trametinib. Bimetinib is being studied in phase 1 and 2 trials with the CDK4/6 inhibitor as well as in the phase 3 trial NCT01763164 compared with dacarbazine in NRAS mutation positive melanomas.55
Selumetinib is a MEK inhibitor that has been compared with dacarbazine and temozolomide with no significant OS advantage. A novel highly specific inhibitor of MEK, cobimetinib, is currently being studied in combination with BRAF inhibitors.
Combined BRAF and MEK Inhibition
A randomized, double-blind, phase 3 study comparing the combination of dabrafenib and trametinib with dabrafenib and placebo in patients with advanced melanoma with a BRAF V600E mutation was presented at the 2014 American Society of Clinical Oncology meeting. Researchers found that after a median follow-up period of 9 months, there was a significant improvement with the combination in the PFS (9.3 months vs 8.8 months) and the ORR (67% vs 51%), with a similar incidence of AEs.56 The combination therapy group had fewer incidences of SCC of the skin but more incidence of pyrexia.
The combination of dabrafenib and trametinib was compared with vemurafenib monotherapy in a recent randomized phase 3 trial among 704 metastatic melanoma patients with a BRAF V600 mutation. Median PFS and ORR were significantly better with combination therapy compared with vemurafenib alone (11.4 months vs 7.3 months, 64% vs 51%, respectively). Overall survival rate at 1 year was significantly improved in the combination group as well (72% vs 65%).57 The incidence of SCC and keratoacanthoma was less in the combination (1%) compared with vemurafenib alone (18%). Another study investigating the coadministration and sequential administration of vemurafenib and trametinib is underway.58
The vemurafenib and cobimetinib combination was studied in a phase 3 trial of previously untreated unresectable locally advanced or metastatic BRAF V600
mutation-positive melanoma. The median PFS was 9.9 months in the combination group and 6.2 months in the control group. The interim analysis showed a 9-month survival rate of 81% in the combination group and 73% in the control group, with no significantly higher incidence of AEs in either arm.59 A longer follow-up will be needed to assess the OS benefit with the combination.
Encorafenib, a selective BRAF inhibitor, has been studied in a phase 1 trial in combination with binimetinib.60 This trial has paved the way to the initiation of a currently ongoing phase 3 trial (NCT01909453) comparing the combination with vemurafenib or encorafenib alone.
C-KIT Inhibitors
Mutations of c-KIT are seen more commonly in chronic sun damage-induced cutaneous melanomas, along with acral and mucosal melanomas.61,62 Earlier trials involving patients without selection for c-KIT mutation positivity failed to show benefit with imatinib. A single-arm, phase 2 trial of imatinib mesylate in patients with metastatic melanoma harboring the c-KIT mutation, an ORR of 23% was achieved, with a median PFS of 3.5 months.63 Imatinib showed an ORR of 29% in a phase 2 trial of mucosal, acral, and in chronic sun damage-induced melanoma patients with c-KIT amplifications and/or mutations. It was demonstrated that c-KIT amplification alone is not as responsive to imatinib compared with c-KIT mutation, suggesting that all patients with these specific melanomas should be tested for KIT mutation status.64
A second-generation c-KIT inhibitor, nilotinib, has shown some promising results with a favorable AE profile in small phase 2 trials.65,66 However, more clinical research will be needed before definite recommendations on its use in cutaneous melanomas can be made. Currently, its role seems to be limited to the management of acral, mucosal, and chronic sun damage-related melanomas with c-KIT mutations.
Future Directions
Angiogenesis promoters, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, fibroblast growth factor, and interleukin-8, are overexpressed in melanoma. Bevacizumab, an anti-VEGF antibody, has been shown to have some benefit in combination with carboplatin and paclitaxel as a triple therapy.67 However, grade 3 AEs were seen in a portion of patients.
The phosphatidylinositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway has also been studied as a target for melanoma therapy. Everolimus, an mTOR inhibitor, was studied in a phase 2 trial in combination with bevacizumab for treatment of metastatic melanoma. The combination showed improved median PFS and OS with the combination (4 months and 8.6 months, respectively), with 43% of patients alive after 12 months of follow-up.68 This study points to the direction of possible benefits with the combination of anti-VEGF and immunotherapy. A recent study failed to show survival advantage with combination of bevacizumab and temozolomide.69
Buparlisib (BKM120), a PI3K inhibitor, has been shown to have activity in vivo and in vitro against melanoma brain metastases.70 More studies need to be done to assess the possible combination with other established therapies.
Oblimersen is an antisense oligonucleotide that suppresses B-cell lymphoma-2, thereby suppressing its anti-apoptotic effect. The triple combination of oblimersen with temozolomide and albumin-bound paclitaxel has shown to be safe and efficacious in a phase 1 trial, thereby creating a need for further clinical trials.71
Treatment Approach
Systemic therapy for metastatic melanoma depends on several factors, including BRAF mutation status, functional status of the patient, disease burden, and severity of symptoms. Assessing the BRAF mutation status has become an important component in the management of patients with metastatic melanoma. It can help recognize patients who will benefit from molecular targeted therapy. In case of a BRAF-positive melanoma, treatment can be initiated with either immunotherapy or BRAF inhibitors. There are no randomized studies comparing immunotherapy to molecular targeted therapy.
Patients who have good PS and lymph node metastases can be treated initially with IL-2, which has the advantage of inducing cure in a minority of patients but should only be considered in patients with well-preserved organ function who can be monitored in an intensive care setting. On the other hand, patients who have bulky, symptomatic disease and poor PS should be treated initially with BRAF inhibitors. Combination of BRAF and MEK inhibitors can also be used and has an improved PFS and OS with potential to cause early tumor regression. There are studies to suggest suboptimal outcomes in patients who are treated with ipilimumab after progression on a BRAF inhibitor compared with initial treatment with ipilimumab followed by a BRAF inhibitor.72-74 However, all these studies are retrospective and there is no prospective data to suggest the above. BRAF mutation-positive patients who progress on a BRAF inhibitor
can be treated with PD-1 inhibitors.
Patients who do not have a BRAF mutation are unlikely to benefit from a BRAF inhibitor and primarily receive immunotherapy with ipilimumab or IL-2. Whenever possible, such patients should be enrolled in a clinical trial, as they have a poor prognosis. Patients who progress on ipilimumab can be treated with one of the PD-1 inhibitors (pembrolizumab, nivolumab). These PD-L1 inhibitors are still being investigated for use in such situations.
The role of chemotherapy in the management of metastatic melanoma has been limited by numerous studies showing significantly better survival with immunotherapy and molecular targeted therapy. Dacarbazine is the only FDA-approved drug for the treatment of melanoma. Its use is reserved mainly for patients who are not candidates for any of the other therapies available, including enrollment in a clinical trial.
Conclusion
Therapies for metastatic melanoma are in a state of flux. In the past decade, several new therapeutic agents have been introduced for the management of this potentially lethal disease. The treatment of metastatic melanoma has gradually shifted from cytotoxic chemotherapy toward a more individualized treatment that has a definite survival advantage over traditional counterparts. The advent of novel therapies has led to initiation of further studies to determine their role in the treatment of advanced melanoma, singly or in combination with other agents. In addition to evaluating new agents, more studies are needed to compare existing treatment modalities so that definitive treatment protocols can be formulated.
Acknowledgement
The authors would like to thank Felicia Ratnaraj, MD, for her assistance in creating the figures.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.