Tolerability of Tretinoin Lotion 0.05% for Moderate to Severe Acne Vulgaris: A Post Hoc Analysis in a Black Population
Acne vulgaris (acne) is the most common dermatologic disorder seen in black patients. However, data are lacking on the effects of treatments, such as topical retinoids. Acne in black patients is frequently associated with postinflammatory hyperpigmentation (PIH), which can be of greater concern than the patient’s acne and often is the main reason these patients seek a dermatologist consultation. Retinoids can treat both acne and PIH. However, the potential for retinoids to induce an irritant contact dermatitis, which could lead to PIH, is a concern. A lotion formulation of tretinoin was developed to provide an important alternative option to treat acne in black patients who may be sensitive to the irritant effects of other tretinoin formulations or where PIH is a concern.
Practice Points
- Acne vulgaris is the most common dermatologic disorder seen in black patients, though data on treatment effects is lacking.
- Postinflammatory hyperpigmentation (PIH) frequently coexists with acne, and retinoids are known to treat both.
- Tretinoin lotion 0.05% is effective in treating both inflammatory and noninflammatory lesions in black patients with acne and reducing PIH without the irritant contact dermatitis seen with other retinoid formulations.
There were no noticeable differences between treatment groups regarding baseline lesion counts or EGSS. At baseline, the mean number (SD) of inflammatory and noninflammatory lesions was 25.2 (4.87) and 41.1 (16.55), respectively. At baseline, 286 (92.9%) participants had moderate acne (EGSS=3). A higher proportion of male participants (10.1%) had severe acne (EGSS=4) at baseline compared to female participants (5.7%).
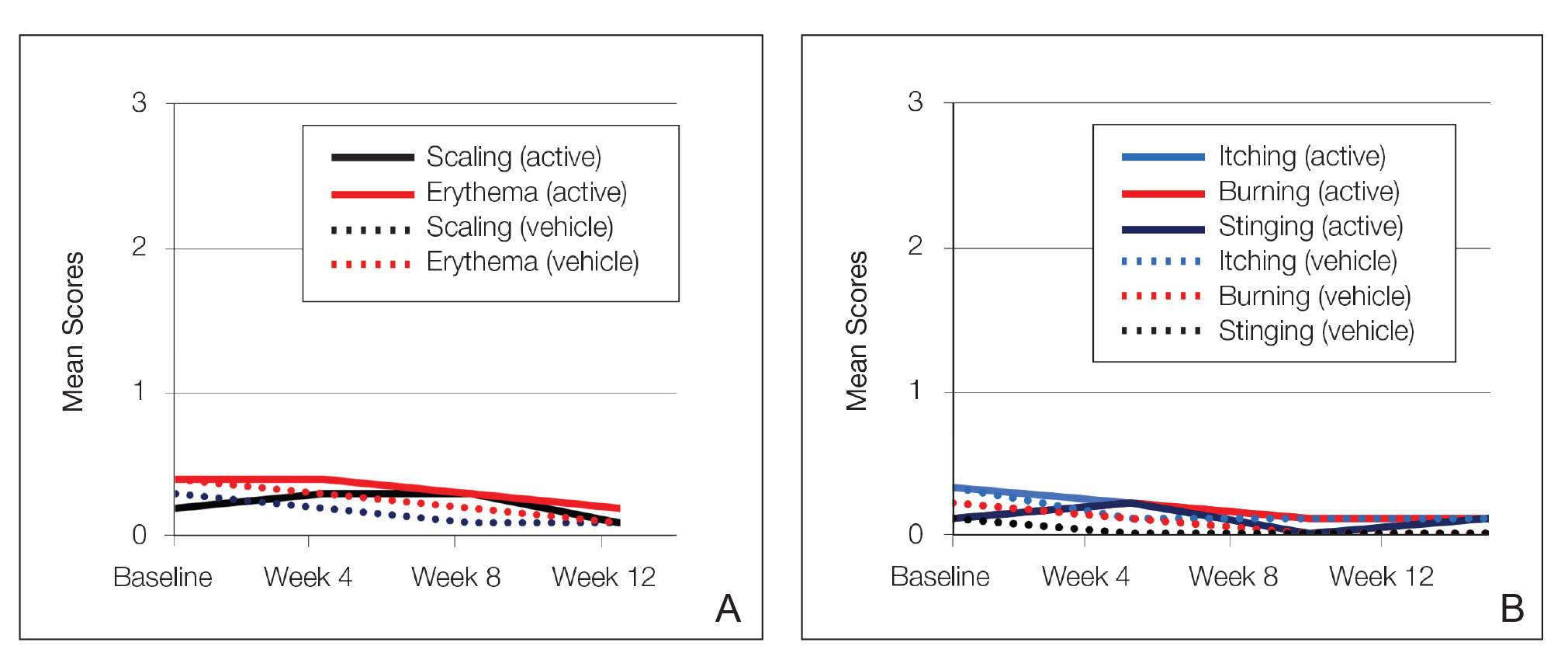
At baseline, the mean score (SD) for scaling, erythema, itching, burning, and stinging in those participants that were subsequently treated with tretinoin lotion 0.05% was 0.2 (0.42), 0.4 (0.68), 0.3 (0.60), 0.1 (0.28), and 0.1 (0.32), respectively (where 1=mild)(Figure 2). There were no differences in mean baseline scores between active and vehicle treatment groups for hyperpigmentation (0.8 each) and hypopigmentation (0.1 each) in the active and vehicle treatment groups. Mean baseline scores were slightly higher in the female participants (0.9) compared to male participants (0.6). Baseline moderate or severe hyperpigmentation was reported in 23.2% and 3.2% of participants, respectively, who were subsequently treated with tretinoin lotion 0.05%, which also was more commonly reported in female participants (33/105 [31.5%]) than male participants (8/50 [16.0%]).
Safety
Treatment-Related AEs
More participants treated with tretinoin lotion 0.05% reported treatment-emergent AEs (TEAEs) compared to vehicle (35 vs 18). The majority of participants reporting TEAEs were female (24 of 35). There were 2 (1.3%) serious AEs with tretinoin lotion 0.05% (both female), and 1 female participant (0.6%) discontinued the study drug because of a TEAE (eTable).
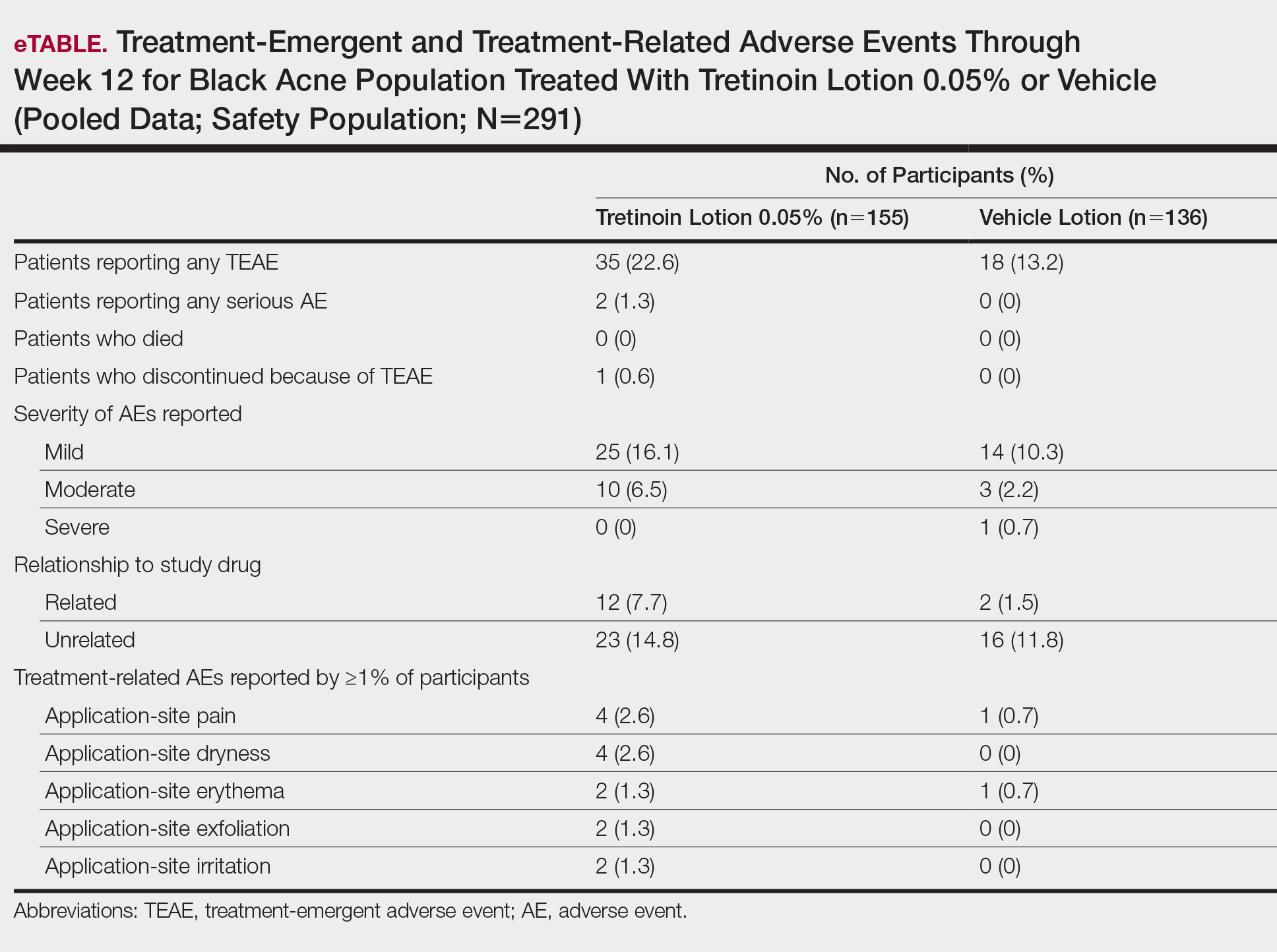
Overall, there were 12 (7.7%) treatment-related AEs; all were mild (n=10) or moderate (n=2). Treatment-related AEs reported by more than 1% of participants treated with tretinoin lotion 0.05% included application-site pain (n=4; 2.6%), dryness (n=4; 2.6%), irritation (n=2; 1.3%), exfoliation (n=2; 1.3%), or erythema (n=2; 1.3%). The majority of treatment-related AEs (10/12) were reported in the female subgroup. Although application-site pain (3.4%) and dryness (3.8%) were more commonly reported in the white population (unpublished data, Ortho Dermatologics) in the 2 studies, differences between the 2 racial groups were not significant.
Cutaneous Safety and Tolerability
Erythema and scaling were recorded by the investigator. Mild to moderate erythema was noted in 31% of participants at baseline, with 21% reporting mild to moderate scaling. Both improved over the study period following treatment with tretinoin lotion 0.05%, with 79% of participants having no erythema and 88% having no scaling by week 12. Mean scores for erythema and scaling remained less than 0.5 throughout the study (1=mild). There were slight transient increases in the mean baseline score for scaling (from 0.2 to 0.3) at week 4 in the active treatment group. By week 12, mean scores were half those reported at baseline (Figure 2).
Severity of itching, burning, and stinging was reported by participants. Overall, 23% reported mild to moderate itching at baseline. Only 7 participants (5%) reported any itching by week 12 in the tretinoin lotion 0.05% group. Reports of burning and stinging were both rare and mild at baseline. Mean scores for itching, burning, and stinging at baseline for those participants who were subsequently treated with tretinoin lotion 0.05% were 0.3, 0.1, and 0.1, respectively (1=mild). Itching severity reduced progressively with treatment. There were slight transient increases in mean scores for burning (from 0.1 to 0.2) and stinging (from 0.1 to 0.2) at week 4, returning to baseline levels or below by week 12.






