Neuromodulatory options for treatment-resistant depression
Evidence is strongest for ECT and rTMS, but other modalities show promise.
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
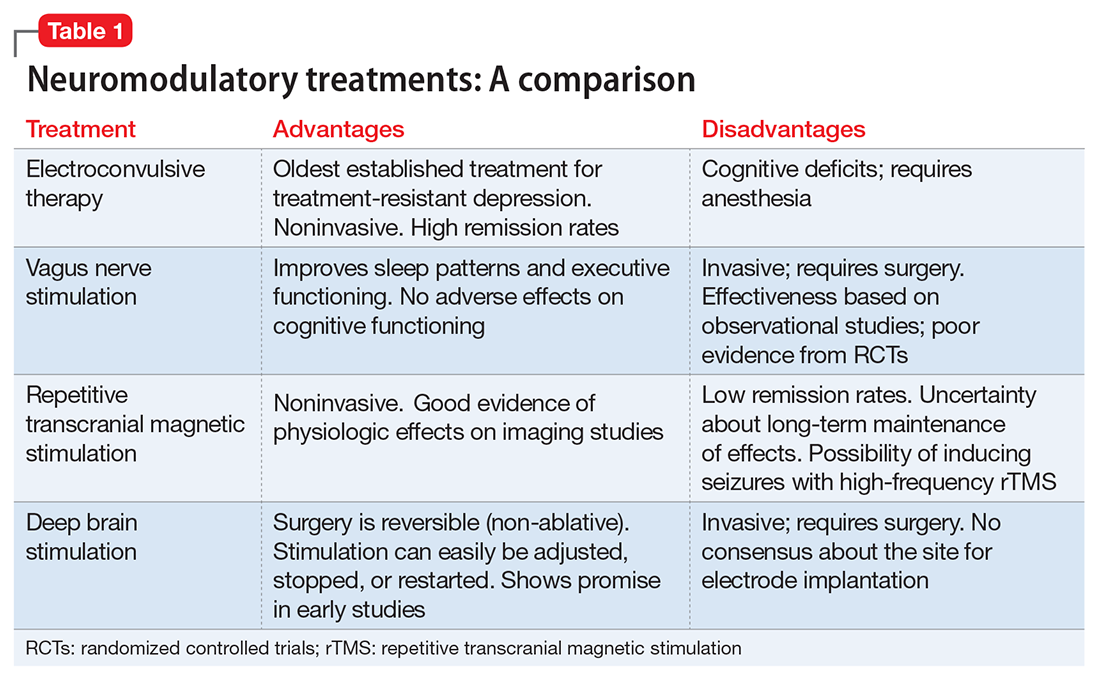
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.
Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
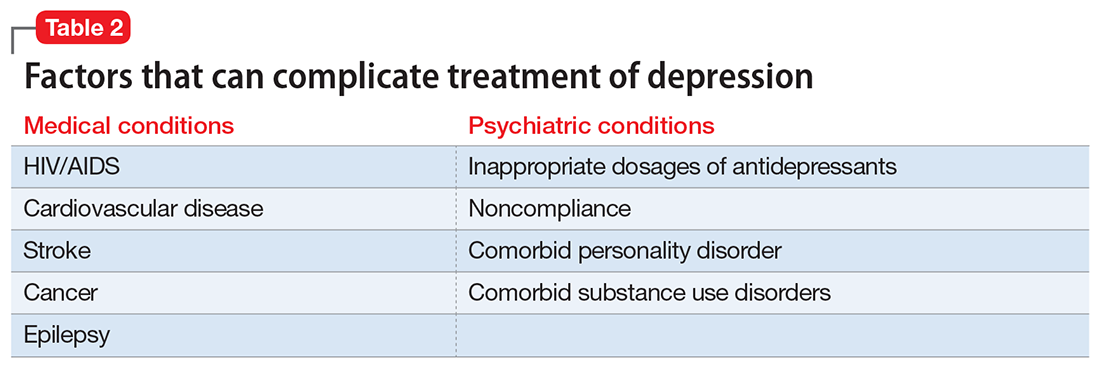
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6
Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11