A Review of Psychostimulants for Adults With Depression
Depression is a common condition that significantly impairs social and occupational functioning. Many patients do not respond to first-line pharmacotherapies and are considered to have treatment-resistant depression (TRD). These patients may benefit from augmentation of their antidepressant to reduce depression. Multiple medications have demonstrated various degrees of efficacy for augmentation, including psychostimulants. This article reviews studies of psychostimulants as augmentation agents for TRD and discusses risks, offers advice, and makes recommendations for clinicians who prescribe stimulants.
Background
Major depressive disorder (MDD) is a common psychiatric condition that significantly impairs quality of life.1 It is a recurrent illness, averaging 2 relapses per decade. The probability of recurrence increases with the number of depressive episodes.2,3 A patient who experiences major depressive episodes alternating with euthymia has unipolar depression; whereas one who experiences major depressive episodes alternating with episodes of mania or hypomania has bipolar depression.4
Despite adequate dose and duration of pharmacotherapy, many individuals with unipolar or bipolar depression do not achieve and sustain remission.5 Remission rates decrease and relapse rates increase with subsequent failed antidepressant trials.6 It is difficult to identify factors that predict treatment resistance, but one review of antidepressant studies found that patients who did not demonstrate a response within 3 weeks of medication initiation were less likely to respond after a longer duration.7
Treatment-resistant depression is commonly, but not universally, defined as lack of response after trials of 2 or more antidepressants with different mechanisms of action for sufficient duration.5 This definition will be used here as well. Other definitions have proposed stages of TRD, but these require further study to evaluate their reliability and predictive utility.8 Due to lack of consensus regarding the definition of TRD, it is not possible to determine the exact prevalence of TRD.
Patients with TRD may benefit from augmentation of their medication regimen. Augmentation with lithium has yielded conflicting results, and its efficacy with newer antidepressants is not well studied.9-12 Triiodothyronine, buspirone, and pindolol have demonstrated some efficacy when added to serotonin reuptake inhibitors (SRIs).10,12,13 Second-generation antipsychotic drugs, antidepressant drug combinations, omega-3 fatty acids, S-adenosyl methionine (SAMe), and L-methylfolate have demonstrated some efficacy in some studies as well.12,14-23 In patients with depression who have not responded to these strategies, psychostimulant augmentation may be appropriate.
Methods
A literature search was conducted following an algorithmic approach in the MEDLINE/PubMed database for studies in English from January 1985 to August 2014 of stimulants as augmenting agents for depression, using the Medical Subject Headings stimulant, depression, and augmentation, combined with an AND operator. The search was limited to adult humans and excluded case reports and letters, to identify studies with stronger evidence. Also excluded were studies using caffeine (to augment electroconvulsive therapy for depression) and pemoline as the sole augmenting stimulant as well as studies of patients with comorbid mental health diagnoses and studies that initiated stimulants and antidepressants simultaneously to assess antidepressant response.
This review organized results by stimulant rather than by depression type, even though some studies used > 1 stimulant or recruited patients with different types of depression. Although prevalence, prognosis, and monotherapy differ for unipolar and bipolar depression, psychostimulants target similar symptoms, despite augmenting different monotherapies in unipolar and bipolar depression. Therefore, no distinction is made between assessing studies of stimulants for unipolar and bipolar depression.
Results
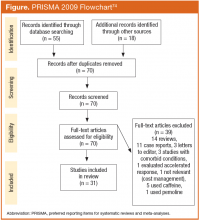
A total of 70 articles were identified, and 31 studies met inclusion criteria (Figure). Of the studies included, 12 were double-blind, placebo-controlled (DBPC) trials and 19 were retrospective chart reviews or open studies. Most studies evaluated depression, using validated scales, such as the Hamilton Depression Rating Scale, Montgomery-Asberg Depression Rating Scale, Clinical Global Impressions of Severity, Inventory of Depressive Symptoms, Carroll Depression Rating Scale, Global Assessment of Functioning, Quick Inventory of Depressive Symptomatology, or the Psychiatric Symptom Assessment Scale. Study details are provided in Tables 1 to 4.
Dextroamphetamine and Methylphenidate
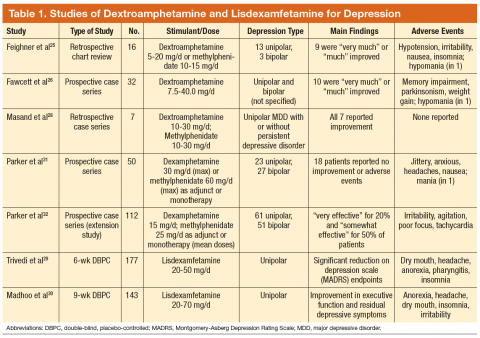
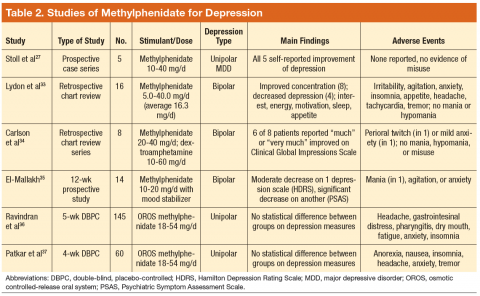
Dextroamphetamine and methylphenidate are indicated for the treatment of attention-deficit/hyperactivity disorder (ADHD) and exert their effects by inhibiting uptake of norepinephrine and dopamine.24 In one chart review, patients received dextroamphetamine or methylphenidate augmentation of monoamine oxidase inhibitors (MAOIs) alone or with concurrent tricyclic antidepressants; the majority reported decreased depression.25 In an openlabel trial, dextroamphetamine was titrated to efficacy in patients who were receiving an MAOI with or without pemoline.26 Nearly 80% of patients reported long-lasting improvement in depression. In an open-label trial, all patients reported decreased depression when methylphenidate was added to SRIs; however, no scales were used.27
In a case series, patients with both major depression and persistent depressive disorder (dysthymia) experienced a substantial, quick, and sustained response to dextroamphetamine or methylphenidate augmentation.28 Addition of lisdexamfetamine significantly reduced depressive symptoms in individuals with inadequate response to escitalopram.29 Patients with full or partial remission of depression noted improved executive function and residual depressive symptoms after lisdexamfetamine was added to SRI monotherapy.30 In a trial in which patients received dexamphetamine or methylphenidate as monotherapy or augmentation, 30% to 34% of patients reported mood improvement, but 36% reported no improvement.31 In an extension study, low-dose psychostimulants quickly diminished melancholia.32
Methylphenidate was safe and effective in patients with bipolar depression receiving treatment for 1 to 5 years; 44% evidenced significant improvement.33 When offered to patients with bipolar depression, patients receiving methylphenidate or dextroamphetamine reported less depression or sedation and did not develop tolerance, mania, or misuse.34 A case series concluded that methylphenidate addition to mood stabilizers was generally effective and safe.35
However, not all preparations of methylphenidate have demonstrated efficacy. In one study, osmotic controlledrelease oral system (OROS) methylphenidate improved apathy and fatigue but not overall depression.36 Although OROS methylphenidate similarly failed to demonstrate statistically significant efficacy in another study, more responders were documented in the treatment group.37
Although this review focuses on stimulants as augmenting agents in patients with depression, it is worth noting the limited number of studies evaluating stimulants’ effect on depression in patients with traumatic brain injury. This observation is of concern, as these conditions are frequently comorbid in returning veterans. One study noted that methylphenidate was an effective monotherapy for depression; whereas another study found that methylphenidate monotherapy reduced depression as well as sertraline, was better tolerated, and improved fatigue and cognition.38,39
Modafinil and Armodafinil
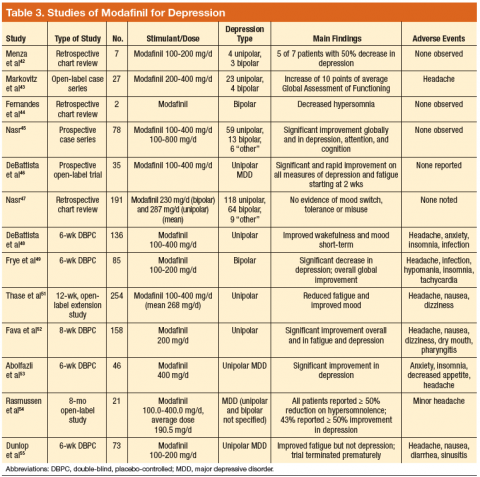
Modafinil and armodafinil (the R-enantiomer of modafinil) are indicated for improving wakefulness in individuals with narcolepsy, obstructive sleep apnea, and shift work sleep disorder by modulating glutamate, gamma amino-butyric acid, and histamine.40,41 Although they increase extracellular dopamine concentrations, they do not cause an increase in dopamine release and may have less misuse potential than that of dextroamphetamine and methylphenidate.40,41 In a study of 7 patients with unipolar or bipolar depression, all patients achieved full or partial remission with minimal adverse effects (AEs).42 In a prospective study, 41% of patients reported only mild depression or full remission with modafinil augmentation.43
Multiple trials and a pooled analysis noted decreased depression and fatigue and improved cognition in patients receiving modafinil augmentation compared with mood stabilizers or antidepressants.44-49 Modafinil is a useful adjunct for partial responders to SRIs, resulting in rapid mood improvement and decreased fatigue.50-54 However, in one study, modafinil did not demonstrate efficacy compared with placebo. This result was attributed to premature study termination after 2 modafinil-treated patients developed suicidal ideation.55 A post hoc analysis found no difference in frequency of suicidal ideation between groups.
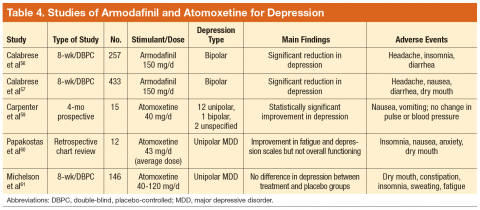
Two DBPC studies evaluated armodafinil in patients with bipolar depression. In both studies it was added to a mood-stabilizing agent (lithium, valproate, aripiprazole, olanzapine, lamotrigine, risperidone, or ziprasidone), and patients receiving armodafinil reported significant reductions
in depression.56,57
Atomoxetine
Atomoxetine is a norepinephrine reuptake inhibitor indicated for the treatment of ADHD and is considered to have no misuse potential due to lack of dopamine modulation.58 In one study, 15 patients received atomoxetine added to their antidepressant, and 60% experienced significant symptom reduction.59 A chart review noted decreases in fatigue and depression when atomoxetine was added to an SRI, mirtazapine, or amitriptyline.60 However, in a DBPC trial, atomoxetine did not lead to significant changes in depression.61
Discussion
There is a limited amount of high-quality evidence to support psychostimulant augmentation, as noted by the relatively few DBPC trials, most of short duration. The evidence supports their efficacy primarily for unipolar depression, as 14 studies evaluated patients with unipolar depression, whereas only 7 studies evaluated patients with bipolar depression. The remaining studies recruited patients with both depression types. Collectively, modafinil and armodafinil have the most evidence in DBPC trials.
There are relatively few DBPC trials with high power and sufficient duration for dextroamphetamine and methylphenidate preparations. This discovery is surprising, considering the duration that these medications have been available. However, several chart reviews and open-label trials provided some evidence to support their use in patients without a history of substance misuse or cardiac conditions.62 Osmotic controlled- release oral system methylphenidate seems to be ineffective, and the efficacy of atomoxetine for augmentation
is uncertain.
Precautions
Prescribing physicians who offer stimulants should consider potential AEs, such as psychosis, anorexia, anxiety, insomnia, mood changes (eg, anger), misuse, addiction, mania, and cardiovascular problems. Psychostimulants have been implicated in precipitating psychosis.63,64 However, in a 12-month study of 250 adults with ADHD, 73 reported AEs, and only 31 discontinued the stimulant. Adverse effects leading to discontinuation included mood instability (n = 7), agitation (n = 6), irritability (n = 4), or decreased appetite (n = 4).65
Although associated with the risks of anorexia and insomnia in patients with ADHD, methylphenidate rapidly improved daytime sleepiness and mood, and—paradoxically—appetite and nighttime sleep in medically ill elderly patients with depression.66 Misuse or abuse of methylphenidate and dextroamphetamine were noted in 23% of patients referred for substance misuse.67 Nonetheless, little evidence exists that these drugs possess significant misuse potential in patients taking them as prescribed. As a prodrug, lisdexamfetamine is hypothesized to have less abuse potential compared with dextroamphetamine and methylphenidate, but it carries the same prescribing and monitoring precautions.68 Risks related to stimulant usage extend to manic symptoms.69 Patients with bipolar disorder should not receive stimulants if they have a history of stimulant-induced mania, rapid cycling, or psychosis.70
Long-term cardiovascular safety data exist for dextroamphetamine and methylphenidate but are limited or unavailable for modafinil, armodafinil, and atomoxetine. A retrospective cohort study found no significant increase in the number of cardiac events in patients receiving dextroamphetamine,
methylphenidate, or atomoxetine for an average of 1 year compared with controls.71 Another cohort study of > 44,000 patients found that initiation of
methylphenidate was associated with increased risk of sudden death or arrhythmia, but the risk was attributed to an unmeasured confounding factor, as the authors found a negative correlation between methylphenidate dose and all cardiovascular events.72
Recent practice guidelines recommend that before prescribing stimulants, clinicians should perform a physical examination (including heart and lung auscultation), obtain vital signs and height and weight, and request an electrocardiogram in case of abnormal findings on a cardiovascular examination or in case of a personal or family history of heart disease. Before offering atomoxetine, clinicians should evaluate the patient for a history of liver disease (and check liver function studies in case of a positive history). Clinicians should also assess risk of self-harm prior to initiating psychostimulant therapy.73 Throughout treatment, clinicians should evaluate the patient for changes in blood pressure, pulse, weight or mood, as well as the development of dependence or misuse. Urine toxicology testing is recommended for dextroamphetamine and methylphenidate to screen for adherence and diversion.
Limitations
Using only PubMed and MEDLINE databases limited the search to articles published in English after 1985, excluding letters and case reports to identify studies with higher evidence (the studies were not weighted based on study design). In addition, the studies had certain limitations. These include a limited number of DBPC trials, most were of short duration. It is also difficult to compare studies due to various rating scales used and concurrent
medication regimens of study subjects. These limitations raise questions surrounding the long-term efficacy of stimulants, and there is no consensus for how long a stimulant should be continued if beneficial. Longer, higherpowered, DBPC trials are warranted to determine longterm efficacy and safety of stimulant augmentation.62
Conclusion
For patients with depression who have not responded to other augmentation strategies, psychostimulants may be offered to improve mood, energy, and concentration. For clinicians considering stimulant augmentation, modafinil and armodafinil are reasonable choices given their efficacy in double-blind, placebo-controlled trials and lower risk of misuse. Dextroamphetamine (particularly lisdexamphetamine) and methylphenidate may be appropriate for patients who have not benefited from or tolerated modafinil or armodafinil, provided these patients do not have a medical history of cardiac disease or current substance use.
Osmotic controlled-release oral system methylphenidate seems to be ineffective as an augmenting agent. The efficacy of atomoxetine for augmentation is questionable, but atomoxetine could be offered if other stimulants were contraindicated, ineffective, or poorly tolerated. Both OROS methylphenidate and atomoxetine should be evaluated in additional trials before they can be recommended as augmentation therapies. Certain psychostimulants may be appropriate and reasonable adjunctive pharmacotherapies for patients with unipolar or bipolar depression who have failed other augmentation strategies, for patients who have significant fatigue or cognitive complaints, or for elderly patients with melancholic or somatic features of depression.
Acknowledgements
The authors thank Maureen Humphrey-Shelton and Kathy Thomas for their help in obtaining references.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.