HPV vaccination coverage among girls is low, CDC reports
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Clinicians can do more to improve human papillomavirus vaccination coverage rates among adolescent females, including emphasizing to parents that the vaccine prevents cancer, according to Dr. Thomas Frieden, the director of the Centers for Disease Control and Prevention.
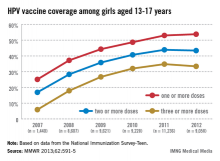
In 2011, only 35% of girls aged 13-17 years had received the full three doses of the HPV vaccine, which dropped slightly in 2012, which is "a huge disappointment," Dr. Frieden said during a July 25 telebriefing. The proportion of adolescent girls who received at least one, two, and the full three-dose HPV vaccine series increased every year from 2007 to 2011, but stalled between 2011 and 2012 and remains at unacceptably low levels – while missed opportunities for vaccination in this population have increased markedly, according to the data (MMWR 2013;62:591-5).
But rates can be increased if clinicians take every possible opportunity to vaccinate adolescents with the HPV vaccine, including when they receive other vaccines, he said during the briefing. While administering a three-dose series was thought to be difficult and was behind the low rates, the data indicated that if the HPV vaccine was given every time the adolescents received another vaccine, the completion of the three-dose series could increase to 93%, he said. According to the report, "Every health care visit, whether for back-to-school evaluations or acute problems, should be used to assess teenagers’ immunization status and provide recommended vaccines if indicated."
"If we get the three-dose series to 80%, an estimated 53,000 cases of cervical cancer could be prevented over the lifetimes of girls aged 12 [years] and younger," Dr. Frieden said.
A three-dose series of Gardasil – the quadrivalent HPV vaccine directed against HPV-16 and -18 (which cause most cervical cancers) and HPV-6 and -11 (which cause most genital warts) – was licensed and recommended by the CDC as a routine vaccine in 2006 for females aged 11-12 years and for females aged 13-26 years who had not been vaccinated. In 2009, the bivalent vaccine Cervarix, directed against HPV-16 and -18, was licensed for use in females aged 10-25 years and added to the recommendation in 2009.
The American Academy of Pediatrics "shares CDC’s disappointment and concern that we are not providing this lifesaving vaccine at the same rates as we do with other immunizations," Dr. Thomas K. McInerny, president of the AAP, said during the briefing.
"Use every opportunity to vaccinate your adolescent patients at both illness and well-child visits," as well as visits for sports physicals, he advised. He also recommended using alerts in electronic medical record systems so vaccination status is reviewed at every patient visit, as well as reminding parents with automated postcards or phone calls, having nurses check vaccine status when they bring patients into the exam room, and implementing standing order policies so that patients can receive vaccines without a physician’s exam or a physician’s order.
The vaccination coverage data were from the National Immunization Survey–Teen (NIS-Teen), which collects data on vaccination among adolescents aged 13-17 years in the 50 states, the District of Columbia, and certain areas of the country, including New York City, and Chicago. Data on HPV vaccination rates among adolescent males will be reported in the future.
In 2007, 25.1% of adolescent girls aged 13-17 years had received at least one dose of the HPV vaccine, which increased to 53% in 2011 and to only 53.8% in 2012. The proportion of adolescent girls who had received the full three-dose series was even lower, increasing from 5.9% in 2007 to 34.8% in 2011 and dropping slightly in 2012 to 33.4%.
The report also provides estimates of missed opportunities for vaccinating adolescent girls, defined as "a health-care encounter occurring on or after a girl’s 11th birthday," and on or after March 23, 2007. Missed opportunities increased from 20.8% in 2007 to 84% in 2012. (March 23, 2007, was when the CDC’s Advisory Committee on Immunization Practices (ACIP) recommendation on the quadrivalent vaccine was published).
The survey found that 23% of parents said they did not plan to get their daughters vaccinated in the next year. The most common reason cited by these parents is that they did not believe the vaccine was necessary (19.1%), followed by concerns about safety (13.1%), vaccine not recommended (14.2%), lack of knowledge of the vaccine or the disease (12.6%), and their daughter was not sexually active (10.1%). Cost did not appear to be an issue.
The report also included safety data, based on the estimated 56 million doses of the quadrivalent vaccine distributed in the United States from June 2006 through March 2013, which did not identify any new safety issues associated with the HPV vaccine, Dr. Frieden said. (Safety focused on the quadrivalent vaccine because most of the vaccine used has been this vaccine).