UPDATE ON OVARIAN CANCER
Neoadjuvant chemotherapy may improve outcomes in women who have bulky, advanced ovarian cancer
IN THIS ARTICLE
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).
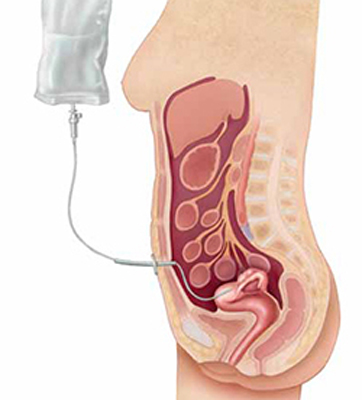
Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.








