UPDATE: MENOPAUSE
Some questions are answered (and some can’t be answered) here about oophorectomy at hysterectomy; nonhormonal agents to ease vasomotor symptoms; and menopausal bone loss
IN THIS ARTICLE
Gabapentin eases symptoms in some women
Along with the antidepressants paroxetine and venlafaxine, gabapentin is the most widely studied prescription agent used in the nonhormonal treatment of menopausal vasomotor symptoms.
Canadian investigators performed a double-blind, placebo-controlled trial involving 197 symptomatic, spontaneously menopausal women (age range: 45 to 65 years). Participants were randomized to gabapentin (300 mg) or placebo, given three times daily for 4 weeks.
Ten of the 99 women who were randomized to gabapentin and six of the 98 women who were randomized to placebo dropped out of the study because of adverse effects. Hot-flash scores decreased by 51% among women taking gabapentin and by 26% among women in the placebo arm (p<.001). Women who used gabapentin were significantly more likely to report dizziness, drowsiness, and unsteadiness than those who received placebo.
Despite its proven efficacy, gabapentin does not appear to equal estrogen in its ability to alleviate vasomotor symptoms. Further, gabapentin’s side-effect profile and the need for thrice-daily administration also limit its appeal. Nevertheless, symptomatic women who are interested in nonhormonal treatment may wish to consider this option.
Anti-osteoporosis arsenal may gain a new weapon
Cummings S, McClung M, Christiansen C, et al. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: results from the FREEDOM Trial. Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; September 16, 2008; Montreal, Canada.
Miller PD, Bolognese MA, Lewiecki EM, et al; AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized, blinded phase 2 clinical trial. Bone. 2008;43:222–229.
In a 3-year study in which more than 7,800 women 60 to 90 years old were randomized to denosumab (60 mg) or placebo, denosumab reduced the risk of vertebral fracture by 68% and the risk of hip fracture by 40%.
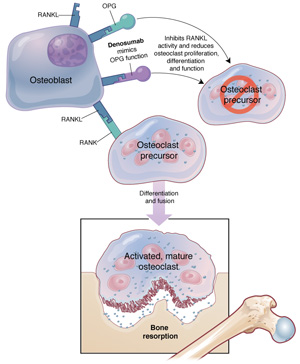
FIGURE A new agent to inhibit bone resorption
RankL is a protein that controls osteoclast maturation, activity, and survival. Denosumab mimics the endogenous decoy receptor OPG to inhibit RankL and reduce osteoclast proliferation, differentiation, and function. Denosumab is a monoclonal antibody to RankL, a mediator of osteoclast function ( FIGURE ). In contrast to alendronate, which enhances trabecular bone density (e.g., that found in the spine), denosumab increases cortical (as in long bones) as well as trabecular bone density.6 If the agent obtains approval by the US Food and Drug Administration, it will be an important new approach to the treatment of osteoporosis in menopausal women.7
The drug is administered every 6 months by subcutaneous injection.
Like estrogen, denosumab has rapidly reversible effects
The trial by Miller and associates found that the effects of denosumab are rapidly reversible after discontinuation of therapy, in contrast to alendronate. As Goldstein noted in “Update on Osteoporosis” in the November 2008 issue of OBG Management, clinicians are still exploring the relationships between alendronate and osteonecrosis of the jaw and proximal femoral-shaft fracture.7 Only further research and surveillance will clarify the clinical pros and cons of denosumab’s swift reversibility.






