What’s the gist of a new FDA label for the LNG-IUS?
Important changes for practice affect insertion and use of the Mirena device, selection of candidates, contraindications, and adverse effects
IN THIS ARTICLE
When should I place the LNG-IUS?
Labeling recommends that, in a cycling woman, the LNG-IUS be placed sometime during the first 7 days of menses. A woman who has it placed at any other time in the cycle needs to be screened for pregnancy and needs to use back-up contraception for 7 days after the LNG-IUS is placed.
How should I place the device?
Complete instructions on insertion are provided in the package insert for the LNG-IUS. A few points about placement highlighted in the new labeling should be noted:
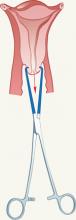
- The need to use a tenaculum to stabilize the cervix and to straighten the uterine axis has been reinforced in the new labeling ( FIGURE 2 )
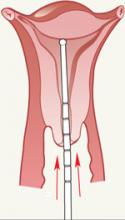
- Careful uterine sounding ( FIGURE 3 ) is needed to evaluate the uterine cavity to rule out any significant distortion of the cavity and to ensure appropriate uterine size before the LNG-IUS package is opened
- Uterine perforation is rare with the LNG-IUS, but to achieve that low risk you must wait at least 10 seconds for the arms of the device to open within the uterine cavity before it is advanced to the fundus
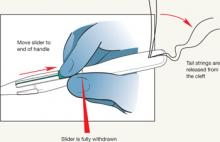
- The procedure-related expulsion rate can be reduced if the clinician moves the slider to the end of the handle (Position 3) carefully and waits for the tail strings to be released from the cleft ( FIGURE 4 ) before trying to withdraw the insertion device.
FIGURE 2 Tenaculum use is key
FIGURE 3 Sound the uterus
FIGURE 4 Release tail strings before withdrawing insertion device
What should I do if the LNG-IUS isn’t at the fundus?
Studies have shown there can be significant migration of the LNG-IUS within the uterine cavity. Fundal placement insures that the tail strings will be long enough to remove the device regardless of where it settles. A device that settles within the lower uterine segment is still effective. Removal of the device is necessary only if 1) a portion of it protrudes from the cervix or 2) the woman has excessive cramping with a low-lying device.
How can I visualize an LNG-IUS?
The LNG-IUS is visible on a radiograph. It is more challenging to image the device by ultrasonography; visualization of the shadowing beneath the device can help localize the unit. It is important to know that the tail strings are more echogenic than the device. Failure to recognize this allows the false impression that the IUD is lower than it actually is.
A woman can safely have a magnetic resonance imaging study without disrupting the position of the LNG-IUS device. It will not set off alarms in security systems—such as those used at an airport
I place only a few LNG-IUS devices each year. What should I do to remain competent and confident at performing this procedure?
Many teaching aids are available to refresh your skills. Options include instructional CD-ROMs and manuals and hands-on practice with a representative of the manufacturer of the device. As noted, the LNG-IUS package insert offers step-by-step instructions on the procedure for placing the device.
What about concerns over cost that some patients express to me?
Women can charge the cost of the device to a credit card if their health insurance does not cover it, or its insertion. Payment plans are also available from the manufacturer.
If you attempt to assist your patients by finding less expensive LNG-IUS units (that is, from a source other than the manufacturer), be aware that the device must be stored under controlled conditions in transit, similar to the way other delicate devices are (e.g., NuvaRing, Implanon). LNG-IUS devices that are shipped under less-than-optimal controlled conditions may not maintain their stability or provide the appropriate rate of release of the contraceptive hormone.