Conservative management of endometrial hyperplasia: New strategies and experimental options
Until recently, hysterectomy was the only alternative for patients with atypical endometrial hyperplasia, the immediate precursor lesion to endometrial cancer, but in recent years a number of organ-sparing treatment possibilities have emerged.
In a trial by Randall and Kurman,8 17 women with atypical endometrial hyperplasia were given either MPA or megestrol acetate for 3 to 12 months. Disease regressed in 16 of these patients—to either no hyperplasia (13 patients) or complex hyperplasia without atypia (3 patients). The remaining patient had persistent atypical hyperplasia after 4 months of treatment with megestrol. One of the women with disease regression subsequently became pregnant and delivered a full-term infant.8
A similar study examined 18 patients with atypical hyperplasia who were treated with MPA for 6 to 12 months.9 Fifteen patients had regression of disease; of these, 4 subsequently became pregnant and delivered full-term infants. One patient treated with MPA for 3 months had progression of disease to well-differentiated adenocarcinoma. The remaining 2 patients had persistent disease despite 3 to 6 months of progestin therapy.9
- Clinical recommendations.
Dosage. Oral megestrol 80 to 120 mg daily or oral MPA 10 to 30 mg daily for approximately 6 months has been shown to cause regression to loss of atypia in 94% of patients with complex atypical hyperplasia and to normal endometrium in 81% of patients.8
Follow-up endometrial sampling. Patients on this regimen should undergo endometrial sampling every 2 to 3 months during therapy until regression to no hyperplasia is evident. Thereafter, sampling should be every 6 to 12 months because recurrence is possible.
When such monitoring is implemented, oral progestin therapy is reasonable, and oral progestin therapy is reasonable, and MPA and megestrol are acceptable choices even in the presence of atypia.
Other routes of administration. A number of investigators have examined the effectiveness of non-oral progestins. Although more studies are needed before their routine clinical use can be advised, the data are encouraging.
- Intramuscular progesterone therapy. In a large prospective study, 192 patients with atypical endometrial hyperplasia were given large weekly doses (500 mg) of intramuscular MPA therapy for 3 months; 96.4% experienced loss of atypia on follow-up curettage specimens.10
- Micronized progesterone vaginal cream was studied in a trial in which 78 patients who had hyperplasia without atypia (60 simple and 18 complex) were treated with cyclic natural micronized progesterone for 3 to 6 months; 90.5% had complete regression of hyperplasia.11
- Levonorgestrel intrauterine devices.Perino et al12 studied the effects of levonorgestrel in 14 patients with endometrial hyperplasia (1 with atypia) over a 1-year period. In all cases without atypia, the hyperplasia regressed within 8 months. The sole patient with atypia experienced regression to hyperplasia without atypia in 2 months.
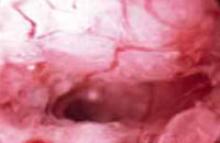
FIGURE 2 Complex hyperplasia with atypia
Photograph taken at hysteroscopy displaying shaggy, irregular endometrium with abnormal blood vessels. Microscopic examination revealed complex endometrial hyperplasia with atypia.
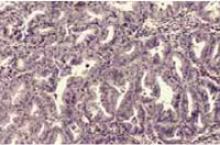
Photomicrograph of the curettage specimen (hematoxylin & eosin×100). At this magnification, complex endometrial hyperplasia with back-to-back glandular crowding is evident.
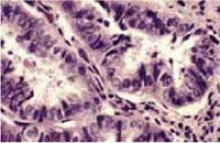
Photomicrograph of the curettage specimen (hematoxylin & eosin×400). Note the heterogeneous nuclei with prominent nucleoli.
Gonadotropin-releasing hormone analogues offer promise
Gonadotropin-releasing hormone (GnRH) analogues suppress the hypothalamicpituitary-ovarian axis, thereby inhibiting estrogen production and, potentially, causing the regression of endometrial hyperplasia. GnRH analogues also appear to have a direct antiproliferative effect on endometrial cells.13
In an investigation of 54 patients given intramuscular GnRH analogues for 6 months, approximately 85% of patients with simple or complex hyperplasia without atypia demonstrated regression to normal endometrium. Of the 3 women with complex atypical hyperplasia, however, only 1 had regression to hyperplasia without atypia, and the other 2 had persistent disease.14
Perez-Medina et al15 gave 19 patients with complex atypical hyperplasia a combination of progestin and GnRH analogues and followed them for 5 years. Patients received intramuscular MPA for 3 months, with 6 months of intramuscular GnRH analogues. Sixteen patients (84.2%) had complete regression at the 5-year follow-up, and 1 patient had persistent disease. One patient had a recurrence of disease at 5 years, and, in another, disease progressed to stage I endometrial adenocarcinoma.15
Clinical recommendations. GnRH analogues appear to be an effective treatment for hyperplasia without atypia, whether simple or complex. At this time, a standardized treatment protocol cannot be recommended without additional data. However, using a GnRH analogue for 6 months with sampling every 3 months is a reasonable option in patients without atypia.
Although the combination of progestin and GnRH agonists increases effectiveness against atypical hyperplasia, and therefore offers promise for patients desiring conservative therapy, existing trials are too small to define a standardized treatment option. Further study is needed before GnRH analogues can be recommended for clinical use in patients with atypical hyperplasia.
Encouraging reports on surgical modalities
Other surgical options have been investigated, but larger studies are needed to determine their safety and efficacy before they can be routinely recommended for women with hyperplasia who refuse hormonal therapy and hysterectomy, or for those who cannot undergo hysterectomy.