HIV management in pregnancy
From preconception gyn care to postpartum follow-up, ObGyns can successfully manage the pregnant patient with HIV using a team-based care model
Third trimester
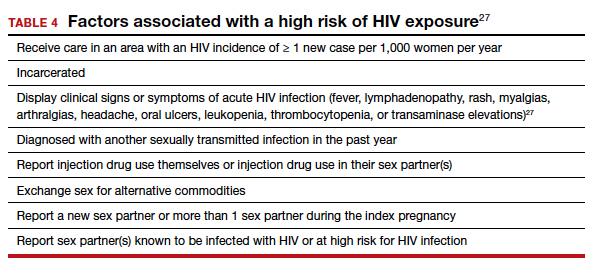
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26
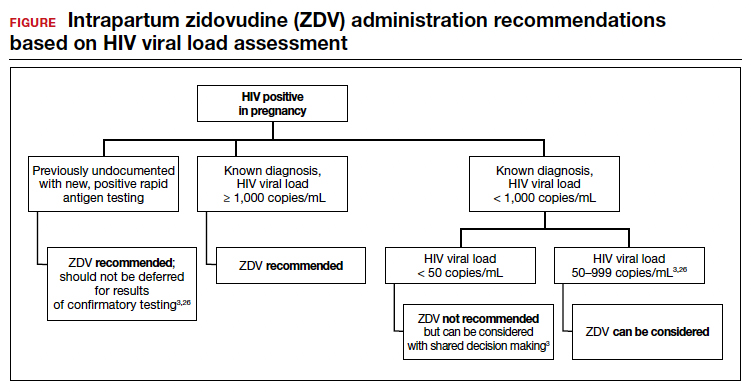
Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).
Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
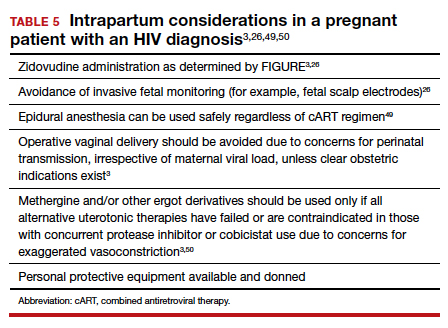
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.
Continue to: Postpartum care...








