The HPV vaccine is now recommended for adults aged 27–45: Counseling implications
Can we improve human papillomavirus (HPV) vaccination rates among boys and girls so “catch-up” vaccinations in adults are unnecessary?
Ali and colleagues studied the preprogram and postprogram incidence of genital warts.9 About 83% received at least 1 dose of vaccine, and 73% of the eligible population completed the 3-dose regimen. There was a significant reduction in warts in both men and women younger than age 21 from 2007 to 2011 (12.1% to 2.2% in men and 11.5% to 0.85% in women). In the 21 to 30 age group there were similar reductions. This study demonstrates that with universal access and public implementation, the rates of HPV-associated disease can be reduced dramatically.
Data informing expanded vaccination ages
Will vaccination of an older population, with presumably many of whom sexually active and at risk for prior exposure to multiple HPV types, have a reasonable impact on lowering HPV-associated cancers? Are HPV-detected lesions in 27- to 45-year-old women the result of reactivation of latent HPV infection, or are they related to new-onset exposure? The FDA reviewed data from 3 studies of HPV vaccination in women aged 27 to 45. The first enrolled women who were naïve to oncogenic HPV types and provided all 3 doses of quadrivalent vaccine were followed for 4 years, along with a comparison group of nonvaccinated women. The second study allowed the nonvaccinated group to receive vaccine in year 4. Both groups were followed up to 10 years with the relevant outcome defined as cumulative incidence of HPV 6/11/16/18-related CIN and condyloma. The third study looked at the same outcomes in a set of all women—whether HPV high-risk naïve or not—after receiving vaccine and followed more than 10 years.7 This last study is most relevant to ObGyns, as it is closest to how we would consider vaccinating our patients.
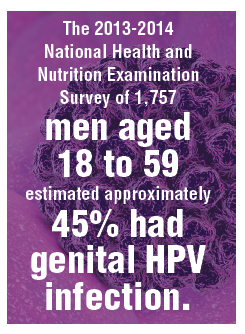
The study findings are reassuring: A large proportion of HPV infections in women between 27 and 45 are the result of new exposure/infection. A study of 420 online daters aged 25 to 65 showed an annual incidence of high-risk HPV types in vaginal swabs of 25.4%, of which 64% were likely new acquisitions.10 The 2013-2014 National Health and Nutrition Examination Survey of 1,757 men aged 18 to 59 estimated approximately 45% had genital HPV infection. There was a bimodal distribution of disease with peaks at 28 to 32 and a larger second peak at 58 to 59 years of age.11 Bottom line: Men and women older than age 26 who are sexually active likely acquire new HPV infections with oncogenic types. Exposure to high-risk HPV types prior to vaccination—as we would expect in the real-world setting—did not eliminate the substantial benefit of immunization.
Based on these study results, and extrapolation to the 9-valent vaccine, the FDA extended the approval of Gardasil 9 to men and women from age 9 to 45. The indications and usage will remain the same: for prevention of cervical, vulvar, vaginal, and anal cancer and genital warts as well as precancerous or dysplastic lesions of the cervix, vulva, vagina, and anus related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Continue to: Impact of the new...






